Targeting envelope proteins of poxviruses to repurpose phytochemicals against monkeypox: An in silico investigation
- PMID: 36687601
- PMCID: PMC9849581
- DOI: 10.3389/fmicb.2022.1073419
Targeting envelope proteins of poxviruses to repurpose phytochemicals against monkeypox: An in silico investigation
Abstract
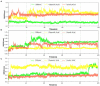
The monkeypox virus (MPXV) has become a major threat due to the increasing global caseload and the ongoing multi-country outbreak in non-endemic territories. Due to limited research in this avenue and the lack of intervention strategies, the present study was aimed to virtually screen bioactive phytochemicals against envelope proteins of MPXV via rigorous computational approaches. Molecular docking, molecular dynamic (MD) simulations, and MM/PBSA analysis were used to investigate the binding affinity of 12 phytochemicals against three envelope proteins of MPXV, viz., D13, A26, and H3. Silibinin, oleanolic acid, and ursolic acid were computationally identified as potential phytochemicals that showed strong binding affinity toward all the tested structural proteins of MPXV through molecular docking. The stability of the docked complexes was also confirmed by MD simulations and MM/PBSA calculations. Results from the iMODS server also complemented the findings from molecular docking and MD simulations. ADME analysis also computationally confirmed the drug-like properties of the phytochemicals, thereby asserting their suitability for consumption. Hence, this study envisions the candidature of bioactive phytochemicals as promising inhibitors against the envelope proteins of the MPXV, serving as template molecules that could further be experimentally evaluated for their efficacy against monkeypox.
Keywords: bioactive phytochemicals; drug repurposing; envelope proteins; molecular docking; molecular simulation; monkeypox virus; poxviruses.
Copyright © 2023 Gulati, Chadha, Harjai and Singh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Development of multi-epitope vaccines against the monkeypox virus based on envelope proteins using immunoinformatics approaches.Front Immunol. 2023 Mar 13;14:1112816. doi: 10.3389/fimmu.2023.1112816. eCollection 2023. Front Immunol. 2023. PMID: 36993967 Free PMC article.
-
Computational studies on searching potential phytochemicals against DNA polymerase activity of the monkeypox virus.J Tradit Complement Med. 2023 May 8;13(5):465-78. doi: 10.1016/j.jtcme.2023.04.002. Online ahead of print. J Tradit Complement Med. 2023. PMID: 37360910 Free PMC article.
-
In silico discovery of potent inhibitors against monkeypox's major structural proteins.J Biomol Struct Dyn. 2023;41(23):14259-14274. doi: 10.1080/07391102.2023.2183342. Epub 2023 Feb 25. J Biomol Struct Dyn. 2023. PMID: 36841550
-
The discovery of novel antivirals for the treatment of mpox: is drug repurposing the answer?Expert Opin Drug Discov. 2023 May;18(5):551-561. doi: 10.1080/17460441.2023.2199980. Epub 2023 Apr 9. Expert Opin Drug Discov. 2023. PMID: 37032577 Review.
-
Monkeypox virus: a re-emergent threat to humans.Virol Sin. 2022 Aug;37(4):477-482. doi: 10.1016/j.virs.2022.07.006. Epub 2022 Jul 9. Virol Sin. 2022. PMID: 35820590 Free PMC article. Review.
Cited by
-
Evaluating the Antiviral Potential of Polyherbal Formulation (Kabasura Kudineer) Against Monkeypox Virus: Targeting E5, Poxin, and DNA Polymerase Through Multifaceted Drug Discovery Approaches.Life (Basel). 2025 May 12;15(5):771. doi: 10.3390/life15050771. Life (Basel). 2025. PMID: 40430198 Free PMC article.
-
Virtual screening and identification of potent phytoconstituents from Acorus calamus L. as inhibitors of Monkeypox virus infection.J Genet Eng Biotechnol. 2025 Jun;23(2):100487. doi: 10.1016/j.jgeb.2025.100487. Epub 2025 Apr 25. J Genet Eng Biotechnol. 2025. PMID: 40390486 Free PMC article.
-
Discovery of a heparan sulfate binding domain in monkeypox virus H3 as an anti-poxviral drug target combining AI and MD simulations.Elife. 2025 Jan 16;13:RP100545. doi: 10.7554/eLife.100545. Elife. 2025. PMID: 39817728 Free PMC article.
-
In silico identification of potential phytochemical inhibitors for mpox virus: molecular docking, MD simulation, and ADMET studies.Mol Divers. 2024 Dec;28(6):4067-4086. doi: 10.1007/s11030-023-10797-2. Epub 2024 Mar 22. Mol Divers. 2024. PMID: 38519803
-
Machine learning-driven docking of diverse DDAs as promising cysteine protease inhibitors targeting Mpox virus.In Silico Pharmacol. 2025 Jun 9;13(2):85. doi: 10.1007/s40203-025-00374-w. eCollection 2025. In Silico Pharmacol. 2025. PMID: 40503562
References
-
- Balkrishna A., Pokhrel S., Singh H., Joshi M., Mulay V. P., Haldar S., et al. (2021). Withanone from Withania somnifera attenuates SARS-CoV-2 RBD and host ACE2 interactions to rescue spike protein induced pathologies in humanized zebrafish model. Drug Des. Devel. Ther. 15, 1111–1133. doi: 10.2147/DDDT.S292805, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

