Colistin potentiation in multidrug-resistant Acinetobacter baumannii by a non-cytotoxic guanidine derivative of silver
- PMID: 36687622
- PMCID: PMC9846554
- DOI: 10.3389/fmicb.2022.1006604
Colistin potentiation in multidrug-resistant Acinetobacter baumannii by a non-cytotoxic guanidine derivative of silver
Abstract
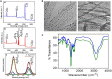
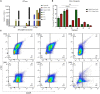
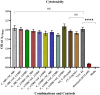
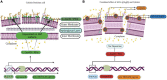
A novel nano-formulation (NF) that sensitizes Acinetobacter baumannii (AB) to otherwise ineffective colistin is described in the present study. Infections due to multidrug resistant (MDR) AB represent a major therapeutic challenge, especially in situations of pre-existing colistin resistance (colR). Subsequently, boosting the effectiveness of colistin would be a better alternative tactic to treat AB infections rather than discovering a new class of antibiotics. We have previously demonstrated an NF comprising self-assembled guanidinium and ionic silver nanoparticles [AD-L@Ag(0)] to have anti-biofilm and bactericidal activity. We report NF AD-L@Ag(0) for the very first time for the potentiation of colistin in Gram-negative colistin-resistant bacteria. Our results implied that a combination of clinically relevant concentrations of colistin and AD-L@Ag(0) significantly decreased colistin-resistant AB bacterial growth and viability, which otherwise was elevated in the presence of only colistin. In this study, we have described various combinations of minimum inhibitory concentration (MIC) of colistin (MICcol, 1/2 MICcol, and 1/4 MICcol) and that of AD-L@Ag(0) [MICAD-L@Ag(0), 1/2 MICAD-L@Ag(0), and 1/4 MICAD-L@Ag(0)] and tested them against MDR AB culture. The results (in broth as well as in solid media) signified that AD-L@Ag(0) was able to potentiate the anti-microbial activity of colistin at sub-MIC concentrations. Furthermore, the viability and metabolic activity of bacterial cells were also measured by CTC fluorescence assay and ATP bioluminescence assay. The results of these assays were in perfect concordance with the scores of cultures (colony forming unit and culture turbidity). In addition, quantitative real-time PCR (qRT-PCR) was performed to unveil the expression of selected genes, DNAgyrA, DNAgyrB, and dac. These genes introduce negative supercoiling in the DNA, and hence are important for basic cellular processes. These genes, due to mutation, modified the Lipid A of bacteria, further resisting the uptake of colistin. Therefore, the expression of these genes was upregulated when AB was treated with only colistin, substantiating that AB is resistant to colistin, whereas the combinations of MICcol + MICAD-L@Ag(0) downregulated the expression of these genes, implying that the developed formulation can potentiate the efficiency of colistin. In conclusion, AD-L@Ag(0) can potentiate the proficiency of colistin, further enhancing colistin-mediated death of AB by putatively disrupting the outer membrane (OM) and facilitating bacterial death.
Keywords: Acinetobacter baumannii; antibiotic; colistin resistance; guanidinium; nano-formulation; potentiators; silver.
Copyright © 2023 Kumar, Singhal, Yadav, Joshi, Patra, Tanwar, Das, Kumar Pramanik and Chaudhuri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
A combination therapy strategy for treating antibiotic resistant biofilm infection using a guanidinium derivative and nanoparticulate Ag(0) derived hybrid gel conjugate.Chem Sci. 2022 Jul 27;13(34):10103-10118. doi: 10.1039/d2sc02980d. eCollection 2022 Aug 31. Chem Sci. 2022. PMID: 36128224 Free PMC article.
-
In vitro activities of ceftazidime/avibactam alone or in combination with antibiotics against multidrug-resistant Acinetobacter baumannii isolates.J Glob Antimicrob Resist. 2019 Jun;17:137-141. doi: 10.1016/j.jgar.2018.12.004. Epub 2018 Dec 18. J Glob Antimicrob Resist. 2019. PMID: 30576787
-
Effects of Sub-Minimum Inhibitory Concentrations of Imipenem and Colistin on Expression of Biofilm-Specific Antibiotic Resistance and Virulence Genes in Acinetobacter baumannii Sequence Type 1894.Int J Mol Sci. 2022 Oct 21;23(20):12705. doi: 10.3390/ijms232012705. Int J Mol Sci. 2022. PMID: 36293559 Free PMC article.
-
Nanotechnology as a therapeutic tool to combat microbial resistance.Adv Drug Deliv Rev. 2013 Nov;65(13-14):1803-15. doi: 10.1016/j.addr.2013.07.011. Epub 2013 Jul 24. Adv Drug Deliv Rev. 2013. PMID: 23892192 Review.
-
Interplay between Colistin Resistance, Virulence and Fitness in Acinetobacter baumannii.Antibiotics (Basel). 2017 Nov 21;6(4):28. doi: 10.3390/antibiotics6040028. Antibiotics (Basel). 2017. PMID: 29160808 Free PMC article. Review.
Cited by
-
Colistin Resistance Mechanism and Management Strategies of Colistin-Resistant Acinetobacter baumannii Infections.Pathogens. 2024 Nov 28;13(12):1049. doi: 10.3390/pathogens13121049. Pathogens. 2024. PMID: 39770308 Free PMC article. Review.
-
The Effect of Semiorganic Iodine-Containing Compounds on the Antibiotic Susceptibility of Pathogenic Microorganisms.Biomedicines. 2025 Jul 22;13(8):1790. doi: 10.3390/biomedicines13081790. Biomedicines. 2025. PMID: 40868046 Free PMC article.
References
-
- Aleshina E. Y., Yudanova T., Skokova I. (2001). Production and properties of polyvinyl alcohol spinning solutions containing protease C and polyhexamethylene guanidine. Fibre Chem. 33 421–423. 10.1023/A:1015039328620 - DOI
-
- Altamimi A. S., El-Azab A. S., Abdelhamid S. G., Alamri M. A., Bayoumi A. H., Alqahtani S. M., et al. (2021). Synthesis, anticancer screening of some novel trimethoxy quinazolines and VEGFR2, EGFR tyrosine kinase inhibitors assay; molecular docking studies. Molecules 26:2992. 10.3390/molecules26102992 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

