CRISPR medicine for blood disorders: Progress and challenges in delivery
- PMID: 36687779
- PMCID: PMC9853164
- DOI: 10.3389/fgeed.2022.1037290
CRISPR medicine for blood disorders: Progress and challenges in delivery
Abstract
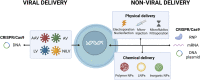
Blood disorders are a group of diseases including hematological neoplasms, clotting disorders and orphan immune deficiency diseases that affects human health. Current improvements in genome editing based therapeutics demonstrated preclinical and clinical proof to treat different blood disorders. Genome editing components such as Cas nucleases, guide RNAs and base editors are supplied in the form of either a plasmid, an mRNA, or a ribonucleoprotein complex. The most common delivery vehicles for such components include viral vectors (e.g., AAVs and RV), non-viral vectors (e.g., LNPs and polymers) and physical delivery methods (e.g., electroporation and microinjection). Each of the delivery vehicles specified above has its own advantages and disadvantages and the development of a safe transferring method for ex vivo and in vivo application of genome editing components is still a big challenge. Moreover, the delivery of genome editing payload to the target blood cells possess key challenges to provide a possible cure for patients with inherited monogenic blood diseases and hematological neoplastic tumors. Here, we critically review and summarize the progress and challenges related to the delivery of genome editing elements to relevant blood cells in an ex vivo or in vivo setting. In addition, we have attempted to provide a future clinical perspective of genome editing to treat blood disorders with possible clinical grade improvements in delivery methods.
Keywords: CRISPR/Cas; blood disorder; non-viral vectors; physical delivery; viral vectors.
Copyright © 2023 Mohammadian Gol, Ureña-Bailén, Hou, Sinn, Antony, Handgretinger and Mezger.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources