In-situ construction of Zr-based metal-organic framework core-shell heterostructure for photocatalytic degradation of organic pollutants
- PMID: 36688034
- PMCID: PMC9845943
- DOI: 10.3389/fchem.2022.1102920
In-situ construction of Zr-based metal-organic framework core-shell heterostructure for photocatalytic degradation of organic pollutants
Abstract
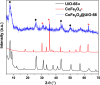
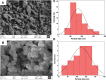
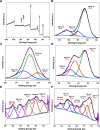
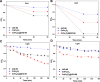
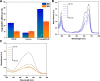
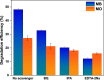
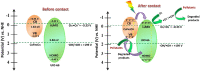
Photocatalysis is an eco-friendly promising approach to the degradation of textile dyes. The majority of reported studies involved remediation of dyes with an initial concentration ≤50 mg/L, which was away from the existing values in textile wastewater. Herein, a simple solvothermal route was utilized to synthesize CoFe2O4@UiO-66 core-shell heterojunction photocatalyst for the first time. The photocatalytic performance of the as-synthesized catalysts was assessed through the photodegradation of methylene blue (MB) and methyl orange (MO) dyes at an initial concentration (100 mg/L). Under simulated solar irradiation, improved photocatalytic performance was accomplished by as-obtained CoFe2O4@UiO-66 heterojunction compared to bare UiO-66 and CoFe2O4. The overall removal efficiency of dyes (100 mg/L) over CoFe2O4@UiO-66 (50 mg/L) reached >60% within 180 min. The optical and photoelectrochemical measurements showed an enhanced visible light absorption capacity as well as effective interfacial charge separation and transfer over CoFe2O4@UiO-66, emphasizing the successful construction of heterojunction. The degradation mechanism was further explored, which revealed the contribution of holes (h+), superoxide (•O2 -), and hydroxyl (•OH) radicals in the degradation process, however, h+ were the predominant reactive species. This work might open up new insights for designing MOF-based core-shell heterostructured photocatalysts for the remediation of industrial organic pollutants.
Keywords: MOFs; core-shell; dyes; ferrite; photocatalysis; visible light.
Copyright © 2023 Abdel Aziz, Sanad, Abdelhameed and Zaki.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









References
-
- Abdi J., Banisharif F., Khataee A. (2021). Amine-functionalized Zr-MOF/CNTs nanocomposite as an efficient and reusable photocatalyst for removing organic contaminants. J. Mol. Liq. 334, 116129. 10.1016/j.molliq.2021.116129 - DOI
-
- Al-Mamun M., Kader S., Islam M., Khan M. (2019). Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 7, 103248. 10.1016/j.jece.2019.103248 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

