Recent advances in functionalized quinoline scaffolds and hybrids-Exceptional pharmacophore in therapeutic medicine
- PMID: 36688036
- PMCID: PMC9859673
- DOI: 10.3389/fchem.2022.1074331
Recent advances in functionalized quinoline scaffolds and hybrids-Exceptional pharmacophore in therapeutic medicine
Abstract
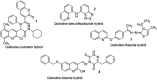
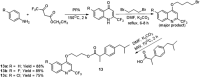
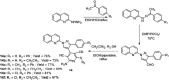
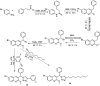
Quinoline is one of the most common nitrogen-containing heterocycles owing to its fascinating pharmacological properties and synthetic value in organic and pharmaceutical chemistry. Functionalization of this moiety at different positions has allowed for varying pharmacological activities of its derivative. Several publications over the last few decades have specified various methods of synthesis. This includes classical methods of synthesizing the primary quinoline derivatives and efficient methods that reduce reaction time with increased yield employing procedures that fulfill one of the twelve green chemistry principles, "safer solvent". The metal nanoparticle-catalyzed reaction also serves as a potent and effective technique for the synthesis of quinoline with excellent atom efficiency. The primary focus of this review is to highlight the routes to synthesizing functionalized quinoline derivatives, including hybrids that have moieties with predetermined activities bound to the quinoline moiety which are of interest in synthesizing drug candidates with dual modes of action, overcoming toxicity, and resistance amongst others. This was achieved using updated literature, stating the biological activities and mechanisms through which these compounds administer relief. The ADMET studies and Structure-Activity Relationship (SAR) of novel derivatives were also highlighted to explore the drug-likeness of the quinoline-hybrids and the influence of substituent characteristics and position on the biological activity of the compounds.
Keywords: admet; drug design; hybrid; pharmacological activity; quinoline; synthesis.
Copyright © 2023 Elebiju, Ajani, Oduselu, Ogunnupebi and Adebiyi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





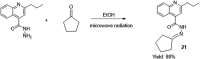
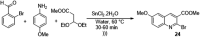









Similar articles
-
Microwave-assisted Synthesis of Pharmacologically Active 4-Phenoxyquinolines and their Benzazole-quinoline Hybrids Through SNAr Reaction of 4,7-dichloroquinoline and Phenols Using [bmim][PF6] as a Green Solvent.Curr Org Synth. 2023;20(5):546-559. doi: 10.2174/1570179419666220830090614. Curr Org Synth. 2023. PMID: 36043752
-
Styrylquinolines Derivatives: SAR Study and Synthetic Approaches.Med Chem. 2022;18(8):859-870. doi: 10.2174/1573406418666220214085856. Med Chem. 2022. PMID: 35156587 Review.
-
Quinolines, a perpetual, multipurpose scaffold in medicinal chemistry.Bioorg Chem. 2021 Apr;109:104639. doi: 10.1016/j.bioorg.2021.104639. Epub 2021 Jan 8. Bioorg Chem. 2021. PMID: 33618829 Review.
-
Synthesis of quinoline mimics via C-H bond functionalization of quinoline: a review on recent progress.Org Biomol Chem. 2025 Mar 12;23(11):2572-2585. doi: 10.1039/d4ob02013h. Org Biomol Chem. 2025. PMID: 39950444 Review.
-
Friedlӓnder's synthesis of quinolines as a pivotal step in the development of bioactive heterocyclic derivatives in the current era of medicinal chemistry.Chem Biol Drug Des. 2022 Dec;100(6):1042-1085. doi: 10.1111/cbdd.14044. Epub 2022 Apr 7. Chem Biol Drug Des. 2022. PMID: 35322543 Review.
Cited by
-
Evaluating Quinolines: Molecular Dynamics Approach to Assess Their Potential as Acetylcholinesterase Inhibitors for Alzheimer's Disease.Chemphyschem. 2025 Jan 2;26(1):e202400653. doi: 10.1002/cphc.202400653. Epub 2024 Nov 8. Chemphyschem. 2025. PMID: 39301943 Free PMC article.
-
Substituted piperazine conjugated to quinoline-thiosemicarbazide as potent α-glucosidase inhibitors to target hyperglycemia.Sci Rep. 2025 Jan 13;15(1):1871. doi: 10.1038/s41598-024-83917-z. Sci Rep. 2025. PMID: 39805968 Free PMC article.
-
Exploring the potential role of GyrA inhibiting quinoline analog: an in silico study.Sci Rep. 2025 Jul 2;15(1):23136. doi: 10.1038/s41598-025-04409-2. Sci Rep. 2025. PMID: 40594094 Free PMC article.
-
Synthesis and In vitro Biological Evaluation of Quinolone-Based Hydrazones as Potential Antidiabetic Agents Targeting Key Metabolic Enzymes.ACS Omega. 2025 Jul 22;10(30):33712-33730. doi: 10.1021/acsomega.5c04663. eCollection 2025 Aug 5. ACS Omega. 2025. PMID: 40787348 Free PMC article.
-
Quinoline Quest: Kynurenic Acid Strategies for Next-Generation Therapeutics via Rational Drug Design.Pharmaceuticals (Basel). 2025 Apr 22;18(5):607. doi: 10.3390/ph18050607. Pharmaceuticals (Basel). 2025. PMID: 40430428 Free PMC article. Review.
References
-
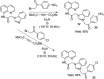
- Abdelbaset M. S., Abdel-Aziz M., Abuo-Rahma G. E. D. A., Abdelrahman M. H., Ramadan M., Youssif B. G. M. (2018). Novel quinoline derivatives carrying nitrones/oximes nitric oxide donors: Design, synthesis, antiproliferative and caspase-3 activation activities. Arch. Pharm. 352 (1), 1800270. 10.1002/ardp.201800270 - DOI - PubMed
-
- Ajani O. O., Iyaye K. T., Audu O. Y., Olorunshola S. J., Kuye A. O., Olanrewaju I. O. (2018). Microwave assisted synthesis and antimicrobial potential of quinoline-based 4-hydrazide-hydrazone derivatives. J. Heterocycl. Chem. 55 (1), 302–312. 10.1002/jhet.3050 - DOI
-
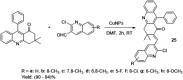
- Angajala G., Aruna V., Subashini R. (2020). An efficient nano-copper catalyzed base-free Knoevenagel condensation: A facile synthesis, molecular modelling simulations, SAR and hypoglycemic studies of new quinoline tethered acridine analogues as PPARγ agonists. J. Mol. Struct. 1220, 128601. 10.1016/j.molstruc.2020.128601 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

