Aptamer-based rapid diagnosis for point-of-care application
- PMID: 36688097
- PMCID: PMC9847464
- DOI: 10.1007/s10404-022-02622-3
Aptamer-based rapid diagnosis for point-of-care application
Abstract
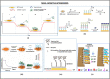
Aptasensors have attracted considerable interest and widespread application in point-of-care testing worldwide. One of the biggest challenges of a point-of-care (POC) is the reduction of treatment time compared to central facilities that diagnose and monitor the applications. Over the past decades, biosensors have been introduced that offer more reliable, cost-effective, and accurate detection methods. Aptamer-based biosensors have unprecedented advantages over biosensors that use natural receptors such as antibodies and enzymes. In the current epidemic, point-of-care testing (POCT) is advantageous because it is easy to use, more accessible, faster to detect, and has high accuracy and sensitivity, reducing the burden of testing on healthcare systems. POCT is beneficial for daily epidemic control as well as early detection and treatment. This review provides detailed information on the various design strategies and virus detection methods using aptamer-based sensors. In addition, we discussed the importance of different aptamers and their detection principles. Aptasensors with higher sensitivity, specificity, and flexibility are critically discussed to establish simple, cost-effective, and rapid detection methods. POC-based aptasensors' diagnostic applications are classified and summarised based on infectious and infectious diseases. Finally, the design factors to be considered are outlined to meet the future of rapid POC-based sensors.
Keywords: Aptamer; Biosensors; Diagnosis; Point of care.
© The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature 2023, Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.Conflicts of interestThe author has no finical and non-financial interests to disclose.
Figures



References
-
- Abrego-Martinez JC, Jafari M, Chergui S, Pavel C, Che D, Siaj M. Aptamer-based electrochemical biosensor for rapid detection of SARS-CoV-2: nanoscale electrode-aptamer-sars-cov-2 imaging by photo-induced force microscopy. Biosens Bioelectron. 2022;195:113595. doi: 10.1016/j.bios.2021.113595. - DOI - PMC - PubMed
-
- Afzal A, Mujahid A, Schirhagl R, Bajwa S, Latif U, Feroz S. Gravimetric viral diagnostics: QCM based biosensors for early detection of viruses. Chemosensors. 2017;5:7. doi: 10.3390/chemosensors5010007. - DOI
-
- Altintas Z, Tothill I. Biomarkers and biosensors for the early diagnosis of lung cancer. Sens Actuators B Chem. 2013;188:988–998. doi: 10.1016/j.snb.2013.07.078. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
