Interactions between amyloid, amyloid precursor protein, and mitochondria
- PMID: 36688439
- PMCID: PMC9987971
- DOI: 10.1042/BST20220518
Interactions between amyloid, amyloid precursor protein, and mitochondria
Abstract
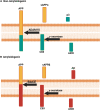
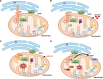
Mitochondrial dysfunction and Aβ accumulation are hallmarks of Alzheimer's disease (AD). Decades of research describe a relationship between mitochondrial function and Aβ production. Amyloid precursor protein (APP), of which Aβ is generated from, is found within mitochondria. Studies suggest Aβ can be generated in mitochondria and imported into mitochondria. APP and Aβ alter mitochondrial function, while mitochondrial function alters Aβ production from APP. The role these interactions contribute to AD pathology and progression are unknown. Here, we discuss prior research, the rigor of those studies, and the critical knowledge gaps of relationships between APP, Aβ, and mitochondria.
Keywords: Alzheimer's disease; amyloid beta; amyloid precursor protein; bioenergetics; mitochondria; γ-secretase.
© 2023 The Author(s).
Conflict of interest statement
The author declares that there are no competing interests associated with this manuscript.
Figures
Similar articles
-
Amyloid precursor protein and mitochondria.Curr Opin Neurobiol. 2023 Feb;78:102651. doi: 10.1016/j.conb.2022.102651. Epub 2022 Nov 30. Curr Opin Neurobiol. 2023. PMID: 36462447 Free PMC article. Review.
-
Amyloid precursor protein processing and bioenergetics.Brain Res Bull. 2017 Jul;133:71-79. doi: 10.1016/j.brainresbull.2016.08.009. Epub 2016 Aug 18. Brain Res Bull. 2017. PMID: 27545490 Free PMC article. Review.
-
The reciprocal relationship between amyloid precursor protein and mitochondrial function.J Neurochem. 2024 Sep;168(9):2275-2284. doi: 10.1111/jnc.16183. Epub 2024 Jul 18. J Neurochem. 2024. PMID: 39022868 Free PMC article. Review.
-
Metabolic Characterization of Intact Cells Reveals Intracellular Amyloid Beta but Not Its Precursor Protein to Reduce Mitochondrial Respiration.PLoS One. 2016 Dec 22;11(12):e0168157. doi: 10.1371/journal.pone.0168157. eCollection 2016. PLoS One. 2016. PMID: 28005987 Free PMC article.
-
Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer's disease.Hum Mol Genet. 2012 Dec 1;21(23):5131-46. doi: 10.1093/hmg/dds360. Epub 2012 Aug 27. Hum Mol Genet. 2012. PMID: 22926141 Free PMC article.
Cited by
-
[Research progress of metalloporphyrin against neurodegene-rative diseases].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Jan 25;54(1):81-89. doi: 10.3724/zdxbyxb-2024-0208. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 39608792 Free PMC article. Review. Chinese.
-
Biomarkers associated with the pathogenesis of Alzheimer's disease.Front Cell Neurosci. 2023 Dec 7;17:1279046. doi: 10.3389/fncel.2023.1279046. eCollection 2023. Front Cell Neurosci. 2023. PMID: 38130871 Free PMC article. Review.
-
Interactions of Cellular Energetic Gene Clusters in the Alzheimer's Mouse Brain.Mol Neurobiol. 2024 Jan;61(1):476-486. doi: 10.1007/s12035-023-03551-0. Epub 2023 Aug 26. Mol Neurobiol. 2024. PMID: 37632678 Free PMC article.
-
Mitochondrial Complex I and β-Amyloid Peptide Interplay in Alzheimer's Disease: A Critical Review of New and Old Little Regarded Findings.Int J Mol Sci. 2023 Nov 3;24(21):15951. doi: 10.3390/ijms242115951. Int J Mol Sci. 2023. PMID: 37958934 Free PMC article. Review.
-
Rescue of protein dyshomeostasis in hippocampal astrocytes from an Alzheimer's disease mouse model by stabilizing ER-mitochondrial interactions at a 20 nm distance.Alzheimers Res Ther. 2025 Jul 4;17(1):148. doi: 10.1186/s13195-025-01793-9. Alzheimers Res Ther. 2025. PMID: 40615914 Free PMC article.
References
-
- Committee NS. (2020) Coding Guidebook for the NACC Neuropathology Data Form. 11
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

