Harnessing the potential of oxygen-generating materials and their utilization in organ-specific delivery of oxygen
- PMID: 36688522
- PMCID: PMC10015602
- DOI: 10.1039/d2bm01329k
Harnessing the potential of oxygen-generating materials and their utilization in organ-specific delivery of oxygen
Abstract
The limited availability of transplantable organs hinders the success of patient treatment through organ transplantation. In addition, there are challenges with immune rejection and the risk of disease transmission when receiving organs from other individuals. Tissue engineering aims to overcome these challenges by generating functional three-dimensional (3D) tissue constructs. When developing tissues or organs of a particular shape, structure, and size as determined by the specific needs of the therapeutic intervention, a tissue specific oxygen supply to all parts of the tissue construct is an utmost requirement. Moreover, the lack of a functional vasculature in engineered tissues decreases cell survival upon implantation in the body. Oxygen-generating materials can alleviate this challenge in engineered tissue constructs by providing oxygen in a sustained and controlled manner. Oxygen-generating materials can be incorporated into 3D scaffolds allowing the cells to receive and utilize oxygen efficiently. In this review, we present an overview of the use of oxygen-generating materials in various tissue engineering applications in an organ specific manner as well as their potential use in the clinic.
Figures
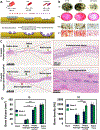
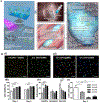
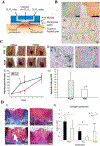
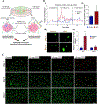

References
-
- Morrison E, Suvarnapathaki S, Blake L, Camci-Unal G. Unconventional biomaterials for cardiovascular tissue engineering. Current Opinion in Biomedical Engineering. 2021;17:100263.
-
- Gómez MP, Pérez B, Manyalich M. International Registry in Organ Donation and Transplantation—2013. Transplantation Proceedings. 2014;46(4):1044–8. - PubMed
-
- Sadeghi F, Ramezani M, Beigee FS, Shadnia S, Moghaddam HH, Zamani N, et al. Organ Procurement From Poisoned Patients: A 14-Year Survey in 2 Academic Centers. Exp Clin Transplant. 2021. - PubMed
-
- Langer R, Vacanti Joseph P. Tissue Engineering. Science. 1993;260(5110):920–6. - PubMed
-
- Bioengineering NioBIa. Tissue Engineering and Regenerative Medicine. In: Services USDoHaH, editor. https://wwwnibibnihgov/sites/default/files/2020-06/Tissue_Engineering_Fa.... Bethesda, MD: National Institutes of Health; 2019. p. 1–2.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

