The Epstein-Barr Virus Glycoprotein BDLF2 Is Essential for Efficient Viral Spread in Stratified Epithelium
- PMID: 36688650
- PMCID: PMC9972961
- DOI: 10.1128/jvi.01528-22
The Epstein-Barr Virus Glycoprotein BDLF2 Is Essential for Efficient Viral Spread in Stratified Epithelium
Abstract
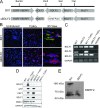
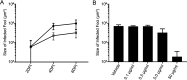
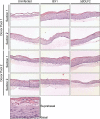
Epstein-Barr virus (EBV) is a ubiquitous human pathogen that infects the majority of the adult population regardless of socioeconomic status or geographical location. EBV primarily infects B and epithelial cells and is associated with different cancers of these cell types, such as Burkitt lymphoma and nasopharyngeal carcinoma. While the life cycle of EBV in B cells is well understood, EBV infection within epithelium is not, largely due to the inability to model productive replication in epithelium in vitro. Organotypic cultures generated from primary human keratinocytes can model many aspects of EBV infection, including productive replication in the suprabasal layers. The EBV glycoprotein BDLF2 is a positional homologue of the murine gammaherpesvirus-68 protein gp48, which plays a role in intercellular spread of viral infection, though sequence homology is limited. To determine the role that BDLF2 plays in EBV infection, we generated a recombinant EBV in which the BDLF2 gene has been replaced with a puromycin resistance gene. The ΔBDLF2 recombinant virus infected both B cell and HEK293 cell lines and was able to immortalize primary B cells. However, the loss of BDLF2 resulted in substantially fewer infected cells in organotypic cultures compared to wild-type virus. While numerous clusters of infected cells representing a focus of infection are observed in wild-type-infected organotypic cultures, the majority of cells observed in the absence of BDLF2 were isolated cells, suggesting that the EBV glycoprotein BDLF2 plays a major role in intercellular viral spread in stratified epithelium. IMPORTANCE The ubiquitous herpesvirus Epstein-Barr virus (EBV) is associated with cancers of B lymphocytes and epithelial cells and is primarily transmitted in saliva. While several models exist for analyzing the life cycle of EBV in B lymphocytes, models of EBV infection in the epithelium have more recently been established. Using an organotypic culture model of epithelium that we previously determined accurately reflects EBV infection in situ, we have ascertained that the loss of the viral envelope protein BDLF2 had little effect on the EBV life cycle in B cells but severely restricted the number of infected cells in organotypic cultures. Loss of BDLF2 has a substantial impact on the size of infected areas, suggesting that BDLF2 plays a specific role in the spread of infection in stratified epithelium.
Keywords: Epstein-Barr virus; organotypic cultures; stratified epithelium; viral spread.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
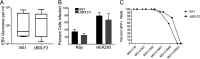


Similar articles
-
Efficient replication of Epstein-Barr virus in stratified epithelium in vitro.Proc Natl Acad Sci U S A. 2014 Nov 18;111(46):16544-9. doi: 10.1073/pnas.1400818111. Epub 2014 Oct 13. Proc Natl Acad Sci U S A. 2014. PMID: 25313069 Free PMC article.
-
New insight into Epstein-Barr virus infection using models of stratified epithelium.PLoS Pathog. 2023 Jan 11;19(1):e1011040. doi: 10.1371/journal.ppat.1011040. eCollection 2023 Jan. PLoS Pathog. 2023. PMID: 36630458 Free PMC article.
-
Generation and Infection of Organotypic Cultures with Epstein-Barr Virus.Methods Mol Biol. 2017;1532:65-78. doi: 10.1007/978-1-4939-6655-4_4. Methods Mol Biol. 2017. PMID: 27873267
-
Epstein-Barr virus latency: LMP2, a regulator or means for Epstein-Barr virus persistence?Adv Cancer Res. 2000;79:175-200. doi: 10.1016/s0065-230x(00)79006-3. Adv Cancer Res. 2000. PMID: 10818681 Review.
-
EBV Infection and Glucose Metabolism in Nasopharyngeal Carcinoma.Adv Exp Med Biol. 2017;1018:75-90. doi: 10.1007/978-981-10-5765-6_6. Adv Exp Med Biol. 2017. PMID: 29052133 Review.
Cited by
-
Molecular Pathogenesis of Human Immunodeficiency Virus-Associated Disease of Oropharyngeal Mucosal Epithelium.Biomedicines. 2023 May 14;11(5):1444. doi: 10.3390/biomedicines11051444. Biomedicines. 2023. PMID: 37239115 Free PMC article. Review.
-
Conditional Cell Reprogramming and Air-Liquid Interface Modeling Life Cycle of Oncogenic Viruses (HPV and EBV) in Epithelial Cells and Virus-Associated Human Carcinomas.Viruses. 2023 Jun 17;15(6):1388. doi: 10.3390/v15061388. Viruses. 2023. PMID: 37376685 Free PMC article. Review.
-
Research landmarks on the 60th anniversary of Epstein-Barr virus.Sci China Life Sci. 2025 Feb;68(2):354-380. doi: 10.1007/s11427-024-2766-0. Epub 2024 Nov 4. Sci China Life Sci. 2025. PMID: 39505801 Review.
-
Oral hairy leukoplakia: The role of Epstein-Barr virus, the occurrence in immunocompetent patients, and the malignant transformation potential.J Dent Sci. 2025 Jul;20(3):1398-1405. doi: 10.1016/j.jds.2024.09.015. Epub 2024 Sep 30. J Dent Sci. 2025. PMID: 40654445 Free PMC article. Review.
-
Understanding the Neurotrophic Virus Mechanisms and Their Potential Effect on Systemic Lupus Erythematosus Development.Brain Sci. 2024 Jan 6;14(1):59. doi: 10.3390/brainsci14010059. Brain Sci. 2024. PMID: 38248274 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

