Minimal Antigenic Evolution after a Decade of Norovirus GII.4 Sydney_2012 Circulation in Humans
- PMID: 36688654
- PMCID: PMC9973034
- DOI: 10.1128/jvi.01716-22
Minimal Antigenic Evolution after a Decade of Norovirus GII.4 Sydney_2012 Circulation in Humans
Abstract
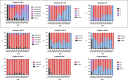
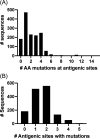
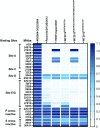
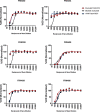
Norovirus is a major human pathogen that can cause severe gastroenteritis in vulnerable populations. The extensive viral diversity presented by human noroviruses constitutes a major roadblock for the development of effective vaccines. In addition to the large number of genotypes, antigenically distinct variants of GII.4 noroviruses have chronologically emerged over the last 3 decades. The last variant to emerge, Sydney_2012, has been circulating at high incidence worldwide for over a decade. We analyzed 1449 capsid sequences from GII.4 Sydney_2012 viruses to determine genetic changes indicative of antigenic diversification. Phylogenetic analyses show that Sydney_2012 viruses scattered within the tree topology with no single cluster dominating during a given year or geographical location. Fourteen residues presented high variability, 7 of which mapped to 4 antigenic sites. Notably, ~52% of viruses presented mutations at 2 or more antigenic sites. Mutational patterns showed that residues 297 and 372, which map to antigenic site A, changed over time. Virus-like particles (VLPs) developed from wild-type Sydney_2012 viruses and engineered to display all mutations detected at antigenic sites were tested against polyclonal sera and monoclonal antibodies raised against Sydney_2012 and Farmington_Hills_2002 VLPs. Minimal changes in reactivity were detected with polyclonal sera and only 4 MAbs lost binding, with all mapping to antigenic site A. Notably, reversion of residues from Sydney_2012 reconstituted epitopes from ancestral GII.4 variants. Overall, this study demonstrates that, despite circulating for over a decade, Sydney_2012 viruses present minimal antigenic diversification and provides novel insights on the diversification of GII.4 noroviruses that could inform vaccine design. IMPORTANCE GII.4 noroviruses are the major cause of acute gastroenteritis in all age groups. This predominance has been attributed to the continued emergence of phylogenetically discrete variants that escape immune responses to previous infections. The last GII.4 variant to emerge, Sydney_2012, has been circulating at high incidence for over a decade, raising the question of whether this variant is undergoing antigenic diversification without presenting a major distinction at the phylogenetic level. Sequence analyses that include >1400 capsid sequences from GII.4 Sydney_2012 showed changes in 4 out of the 6 major antigenic sites. Notably, while changes were detected in one of the most immunodominant sites over time, these resulted in minimal changes in the antigenic profile of these viruses. This study provides new insights on the mechanism governing the antigenic diversification of GII.4 norovirus that could help in the development of cross-protective vaccines to human noroviruses.
Keywords: antigenic variation; calicivirus; gastroenteritis; norovirus; phylogenetic analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Marionneau S, Ruvoen N, Le Moullac-Vaidye B, Clement M, Cailleau-Thomas A, Ruiz-Palacois G, Huang P, Jiang X, Le Pendu J. 2002. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology 122:1967–1977. 10.1053/gast.2002.33661. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

