Effects of faecal microbiota transplantation on the small intestinal mucosa in systemic sclerosis
- PMID: 36688692
- PMCID: PMC10393441
- DOI: 10.1093/rheumatology/kead014
Effects of faecal microbiota transplantation on the small intestinal mucosa in systemic sclerosis
Abstract
Objectives: In SSc, gastrointestinal tract (GIT) involvement is a major concern, with no disease-modifying and limited symptomatic therapies available. Faecal microbiota transplantation (FMT) represents a new therapeutic option for GIT-affliction in SSc, showing clinical promise in a recent controlled pilot trial. Here, we aim to investigate effects of FMT on duodenal biopsies collected from SSc patients by immunohistochemistry and transcriptome profiling.
Methods: We analysed duodenal biopsies obtained pre-intervention (week 0) and post-intervention (weeks 2 and 16) from nine SSc patients receiving an intestinal infusion of FMT (n = 5) or placebo (n = 4). The analysis included immunohistochemistry (IHC) with a selected immune function and fibrosis markers, and whole biopsy transcriptome profiling.
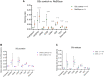
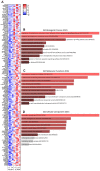
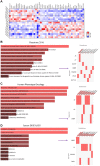
Results: In patients receiving FMT, the number of podoplanin- and CD64-expressing cells in the mucosa were lower at week 2 compared with baseline. This decline in podoplanin- (r = 0.94) and CD64-positive (r = 0.89) cells correlated with improved patient-reported lower GIT symptoms. Whole biopsy transcriptome profiling from week 2 showed significant enrichment of pathways critical for cellular and endoplasmic reticulum stress responses, microvillus and secretory vesicles, vascular and sodium-dependent transport, and circadian rhythm. At week 16, we found enrichment of pathways mandatory for binding activity of immunoglobulin receptors, T cell receptor complexes, and chemokine receptors, as well as response to zinc-ions. We found that 25 genes, including Matrix metalloproteinase-1 were upregulated at both week 2 and week 16.
Conclusion: Combining selective IHC and unbiased gene expression analyses, this exploratory study highlights the potential for disease-relevant organ effects of FMT in SSc patients with GIT involvement.
Trial registration: ClinicalTrials.gov, http://clinicaltrials.gov, NCT03444220.
Keywords: RNA-sequencing; SSc; faecal microbiota transplantations; gastrointestinal tract; immunohistochemistry.
© The Author(s) 2023. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures


References
-
- Thoua NM, Bunce C, Brough G et al. Assessment of gastrointestinal symptoms in patients with systemic sclerosis in a UK tertiary referral centre. Rheumatology 2010;49:1770–5. - PubMed

