Immune-interacting lymphatic endothelial subtype at capillary terminals drives lymphatic malformation
- PMID: 36688917
- PMCID: PMC9884640
- DOI: 10.1084/jem.20220741
Immune-interacting lymphatic endothelial subtype at capillary terminals drives lymphatic malformation
Abstract
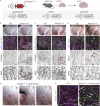
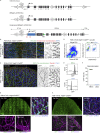
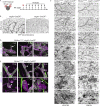
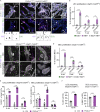
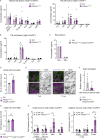
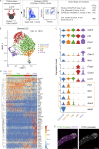
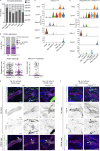
Oncogenic mutations in PIK3CA, encoding p110α-PI3K, are a common cause of venous and lymphatic malformations. Vessel type-specific disease pathogenesis is poorly understood, hampering development of efficient therapies. Here, we reveal a new immune-interacting subtype of Ptx3-positive dermal lymphatic capillary endothelial cells (iLECs) that recruit pro-lymphangiogenic macrophages to promote progressive lymphatic overgrowth. Mouse model of Pik3caH1047R-driven vascular malformations showed that proliferation was induced in both venous and lymphatic ECs but sustained selectively in LECs of advanced lesions. Single-cell transcriptomics identified the iLEC population, residing at lymphatic capillary terminals of normal vasculature, that was expanded in Pik3caH1047R mice. Expression of pro-inflammatory genes, including monocyte/macrophage chemokine Ccl2, in Pik3caH1047R-iLECs was associated with recruitment of VEGF-C-producing macrophages. Macrophage depletion, CCL2 blockade, or anti-inflammatory COX-2 inhibition limited Pik3caH1047R-driven lymphangiogenesis. Thus, targeting the paracrine crosstalk involving iLECs and macrophages provides a new therapeutic opportunity for lymphatic malformations. Identification of iLECs further indicates that peripheral lymphatic vessels not only respond to but also actively orchestrate inflammatory processes.
© 2023 Petkova et al.
Conflict of interest statement
Disclosures: E. Baselga reported a patent for TOPIAL PIK3CA inhibitor issued. M. Graupera reported “other” from ArQule, Inc., a wholly owned subsidiary of Merck & Co., Inc and “other” from Venthera during the conduct of the study. No other disclosures were reported.
Figures
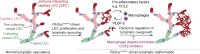







References
-
- Alishekevitz, D., Gingis-Velitski S., Kaidar-Person O., Gutter-Kapon L., Scherer S.D., Raviv Z., Merquiol E., Ben-Nun Y., Miller V., Rachman-Tzemah C., et al. . 2016. Macrophage-induced lymphangiogenesis and metastasis following paclitaxel chemotherapy is regulated by VEGFR3. Cell Rep. 17:1344–1356. 10.1016/j.celrep.2016.09.083 - DOI - PMC - PubMed
-
- di Blasio, L., Puliafito A., Gagliardi P.A., Comunanza V., Somale D., Chiaverina G., Bussolino F., and Primo L.. 2018. PI3K/mTOR inhibition promotes the regression of experimental vascular malformations driven by PIK3CA-activating mutations. Cell Death Dis. 9:45. 10.1038/s41419-017-0064-x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

