Delayed renal injury in survivors of hematologic acute radiation syndrome
- PMID: 36688956
- PMCID: PMC10313734
- DOI: 10.1080/09553002.2023.2170491
Delayed renal injury in survivors of hematologic acute radiation syndrome
Abstract
Purpose: A mass casualty disaster involving radiological or nuclear agents continues to be a public health concern which requires consideration of both acute and late tissue toxicities in exposed victims. With the advent of advanced treatment options for the mitigation of hematological injuries, there are likely to be survivors of total body irradiation (TBI) exposures as high as 8-10 Gy. These survivors are at risk for a range of delayed multi-organ morbidities including progressive renal failure.
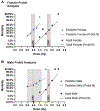
Material and methods: Here, we established the WAG/RijCmcr rat as an effective model for the evaluation of medical countermeasures (MCM) for acute hematologic radiation syndrome (H-ARS). The LD50/30 dose for adult and pediatric WAG/RijCmcr rats was determined for both sexes. We then confirmed the FDA-approved MCM pegfilgrastim (peg-GCSF, Neulasta®) mitigates H-ARS in adult male and female rats. Finally, we evaluated survival and renal dysfunction up to 300 d post-TBI in male and female adult rats.
Results: In the WAG/RijCmcr rat model, 87.5% and 100% of adult rats succumb to lethal hematopoietic acute radiation syndrome (H-ARS) at TBI doses of 8 and 8.5 Gy, respectively. A single dose of the hematopoietic growth factor peg-GCSF administered at 24 h post-TBI improved survival during H-ARS. Peg-GCSF treatment improved 30 d survival from 12.5% to 83% at 8 Gy and from 0% to 63% at 8.5 Gy. We then followed survivors of H-ARS through day 300. Rats exposed to TBI doses greater than 8 Gy had a 26% reduction in survival over days 30-300 compared to rats exposed to 7.75 Gy TBI. Concurrent with the reduction in long-term survival, a dose-dependent impairment of renal function as assessed by blood urea nitrogen (BUN) and urine protein to urine creatinine ratio (UP:UC) was observed.
Conclusion: Together, these data show survivors of H-ARS are at risk for the development of delayed renal toxicity and emphasize the need for the development of medical countermeasures for delayed renal injury.
Keywords: Acute radiation syndrome (ARS); growth factors; haematology – radiation; multiple organ failure (MOF); radiation-induced tissue toxicity; total body irradiation (TBI).
Conflict of interest statement
Figures




References
-
- Hankey KG, Farese AM, Blaauw EC, Gibbs AM, Smith CP, Katz BP, Tong Y, Prado KL, MacVittie TJ. 2015. Pegfilgrastim Improves Survival of Lethally Irradiated Nonhuman Primates. Radiat Res. 183(6):643–655. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
