Deliberate Self-Poisoning: Real-Time Characterization of Suicidal Habits and Toxidromes in the Food and Drug Administration Adverse Event Reporting System
- PMID: 36689131
- PMCID: PMC9869307
- DOI: 10.1007/s40264-022-01269-x
Deliberate Self-Poisoning: Real-Time Characterization of Suicidal Habits and Toxidromes in the Food and Drug Administration Adverse Event Reporting System
Abstract
Introduction: Deliberate self-poisoning (DSP) using drugs is the preferred method of suicide at a global level. Its investigation is hampered by limited sample sizes and data reliability. We investigate the role of the US FDA Adverse Event Reporting System (FAERS), a consolidated pharmacovigilance database, in outlining DSP habits and toxidromes.
Methods: We retrieved cases of 'intentional overdose' and 'poisoning deliberate' from the FAERS (January 2004-December 2021). Using descriptive and disproportionality analyses, we estimated temporal trends, potential risk factors, toxidromes, case-fatality rates and lethal doses (LDs) for the most frequently reported drugs.
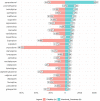
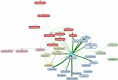
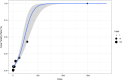
Results: We retrieved 42,103 DSP cases (17% fatal). Most cases were submitted in winter. Reports of DSP involved younger people, psychiatric conditions, and alcohol use, compared with non-DSP, and fatality was higher in men and older patients. Suspected drugs were mainly antidepressants, analgesics, and antipsychotics. Multiple drug intake was recorded in more than 50% of the reports, especially analgesics, psychotropics, and cardiovascular agents. The most frequently reported drugs were paracetamol, promethazine, amlodipine, quetiapine, and metformin. We estimated LD25 for paracetamol (150 g).
Conclusion: Worldwide coverage of the FAERS complements existing knowledge about DSP and may drive tailored prevention measures to timely address the DSP phenomenon and prevent intentional suicides.
© 2023. The Author(s).
Conflict of interest statement
Michele Fusaroli, Guido Pelletti, Valentina Giunchi, Chiara Pugliese, Mattia Bartolucci, Elena Narmine Necibi, Emanuel Raschi, Fabrizio De Ponti, Susi Pelotti, and Elisabetta Poluzzi declare no conflicts of interest in relation to this research.
Figures

Comment in
-
Authors' Reply to Cappello et al. Comment on: "Deliberate Self-Poisoning: Real-Time Characterization of Suicidal Habits and Toxidromes in the Food and Drug Administration Adverse Event Reporting System".Drug Saf. 2023 Sep;46(9):919-920. doi: 10.1007/s40264-023-01331-2. Epub 2023 Aug 12. Drug Saf. 2023. PMID: 37572204 No abstract available.
-
Comment on: "Deliberate Self-Poisoning: Real-Time Characterisation of Suicidal Habits and Toxidromes in the Food and Drug Administration Adverse Event Reporting System".Drug Saf. 2023 Sep;46(9):917-918. doi: 10.1007/s40264-023-01330-3. Epub 2023 Aug 12. Drug Saf. 2023. PMID: 37572205 No abstract available.
Similar articles
-
Comment on: "Deliberate Self-Poisoning: Real-Time Characterisation of Suicidal Habits and Toxidromes in the Food and Drug Administration Adverse Event Reporting System".Drug Saf. 2023 Sep;46(9):917-918. doi: 10.1007/s40264-023-01330-3. Epub 2023 Aug 12. Drug Saf. 2023. PMID: 37572205 No abstract available.
-
Authors' Reply to Cappello et al. Comment on: "Deliberate Self-Poisoning: Real-Time Characterization of Suicidal Habits and Toxidromes in the Food and Drug Administration Adverse Event Reporting System".Drug Saf. 2023 Sep;46(9):919-920. doi: 10.1007/s40264-023-01331-2. Epub 2023 Aug 12. Drug Saf. 2023. PMID: 37572204 No abstract available.
-
Mixed Approach Retrospective Analyses of Suicide and Suicidal Ideation for Brand Compared with Generic Central Nervous System Drugs.Drug Saf. 2018 Apr;41(4):363-376. doi: 10.1007/s40264-017-0624-0. Drug Saf. 2018. PMID: 29196989 Free PMC article.
-
Medication overdose data analysis: a review of medication error reports in the FDA adverse event reporting system (FAERS).BMC Pharmacol Toxicol. 2023 Aug 4;24(1):41. doi: 10.1186/s40360-023-00681-y. BMC Pharmacol Toxicol. 2023. PMID: 37542326 Free PMC article. Review.
-
Prevention of suicide and attempted suicide in Denmark. Epidemiological studies of suicide and intervention studies in selected risk groups.Dan Med Bull. 2007 Nov;54(4):306-69. Dan Med Bull. 2007. PMID: 18208680 Review.
Cited by
-
Comment on: "Deliberate Self-Poisoning: Real-Time Characterisation of Suicidal Habits and Toxidromes in the Food and Drug Administration Adverse Event Reporting System".Drug Saf. 2023 Sep;46(9):917-918. doi: 10.1007/s40264-023-01330-3. Epub 2023 Aug 12. Drug Saf. 2023. PMID: 37572205 No abstract available.
-
An exploratory study evaluating the 20 medications most commonly associated with suicidal ideation and self-injurious behavior in the FAERS database.BMC Pharmacol Toxicol. 2025 Jan 30;26(1):24. doi: 10.1186/s40360-025-00858-7. BMC Pharmacol Toxicol. 2025. PMID: 39885564 Free PMC article.
-
Unveiling the Burden of Drug-Induced Impulsivity: A Network Analysis of the FDA Adverse Event Reporting System.Drug Saf. 2024 Dec;47(12):1275-1292. doi: 10.1007/s40264-024-01471-z. Epub 2024 Aug 15. Drug Saf. 2024. PMID: 39147961 Free PMC article.
-
Suicide risk among adult subjects hospitalized in an acute psychiatric ward: 6-year retrospective investigation.BMC Public Health. 2024 Nov 11;24(1):3113. doi: 10.1186/s12889-024-20450-8. BMC Public Health. 2024. PMID: 39529024 Free PMC article.
-
Adverse events associated with amlodipine: a pharmacovigilance study using the FDA adverse event reporting system.Front Cardiovasc Med. 2025 May 6;12:1504671. doi: 10.3389/fcvm.2025.1504671. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40395720 Free PMC article.
References
-
- Hedegaard H, Curtin SC, Warner M. Suicide mortality in the United States, 1999–2019. NCHS Data Brief. 2021;1–8. - PubMed
-
- Yip PSF, Zheng Y, Wong C. Demographic and epidemiological decomposition analysis of global changes in suicide rates and numbers over the period 1990–2019. Injury prevention. BMJ. 2022;28:117–124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

