Effect of Disposable Elevator Cap Duodenoscopes on Persistent Microbial Contamination and Technical Performance of Endoscopic Retrograde Cholangiopancreatography: The ICECAP Randomized Clinical Trial
- PMID: 36689215
- PMCID: PMC9871945
- DOI: 10.1001/jamainternmed.2022.6394
Effect of Disposable Elevator Cap Duodenoscopes on Persistent Microbial Contamination and Technical Performance of Endoscopic Retrograde Cholangiopancreatography: The ICECAP Randomized Clinical Trial
Abstract
Importance: Infection transmission following endoscopic retrograde cholangiopancreatography (ERCP) can occur due to persistent contamination of duodenoscopes despite high-level disinfection to completely eliminate microorganisms on the instrument.
Objective: To determine (1) contamination rates after high-level disinfection and (2) technical performance of duodenoscopes with disposable elevator caps compared with those with standard designs.
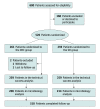
Design, setting, and participants: In this parallel-arm multicenter randomized clinical trial at 2 tertiary ERCP centers in Canada, all patients 18 years and older and undergoing ERCP for any indication were eligible.
Intervention: The intervention was use of duodenoscopes with disposable elevator caps compared with duodenoscopes with a standard design.
Main outcomes and measures: Coprimary outcomes were persistent microbial contamination of the duodenoscope elevator or channel, defined as growth of at least 10 colony-forming units of any organism or any growth of gram-negative bacteria following high-level disinfection (superiority outcome), and technical success of ERCP according to a priori criteria (noninferiority outcome with an a priori noninferiority margin of 7%), assessed by blinded reviewers.
Results: From December 2019 to February 2022, 518 patients were enrolled (259 disposable elevator cap duodenoscopes, 259 standard duodenoscopes). Patients had a mean (SD) age of 60.7 (17.0) years and 258 (49.8%) were female. No significant differences were observed between study groups, including in ERCP difficulty. Persistent microbial contamination was detected in 11.2% (24 of 214) of standard duodenoscopes and 3.8% (8 of 208) of disposable elevator cap duodenoscopes (P = .004), corresponding to a relative risk of 0.34 (95% CI, 0.16-0.75) and number needed to treat of 13.6 (95% CI, 8.1-42.7) to avoid persistent contamination. Technical success using the disposable cap scope was noninferior to that of the standard scope (94.6% vs 90.7%, P = .13). There were no differences between study groups in adverse events and other secondary outcomes.
Conclusions and relevance: In this randomized clinical trial, disposable elevator cap duodenoscopes exhibited reduced contamination following high-level disinfection compared with standard scope designs, without affecting the technical performance and safety of ERCP.
Trial registration: ClinicalTrials.gov Identifier: NCT04040504.
Conflict of interest statement
Figures

Comment in
-
Duodenoscopes With Disposable Elevator Caps-An Incremental Reduction in Infection Risk for Patients.JAMA Intern Med. 2023 Mar 1;183(3):200-202. doi: 10.1001/jamainternmed.2022.6393. JAMA Intern Med. 2023. PMID: 36689242 No abstract available.
-
Disposable Elevator Caps Reduce Microbial Contamination After ERCP.Gastroenterology. 2023 Sep;165(3):801. doi: 10.1053/j.gastro.2023.03.209. Epub 2023 Mar 20. Gastroenterology. 2023. PMID: 36940776 No abstract available.
Similar articles
-
Infection control in ERCP using a duodenoscope with a disposable cap (ICECAP): rationale for and design of a randomized controlled trial.BMC Gastroenterol. 2020 Mar 12;20(1):64. doi: 10.1186/s12876-020-01200-7. BMC Gastroenterol. 2020. PMID: 32164535 Free PMC article.
-
Contamination of Disposable Distal Cap Duodenoscopes and Detachable Elevator Duodenoscopes After Reprocessing: A Randomized Trial.J Gastroenterol Hepatol. 2025 Feb;40(2):520-527. doi: 10.1111/jgh.16827. Epub 2024 Nov 25. J Gastroenterol Hepatol. 2025. PMID: 39586581 Clinical Trial.
-
Bioburden and transmission of pathogenic bacteria through elevator channel during endoscopic retrograde cholangiopancreatography: application of multiple-locus variable-number tandem-repeat analysis for characterization of clonal strains.Expert Rev Med Devices. 2019 May;16(5):413-420. doi: 10.1080/17434440.2019.1604215. Epub 2019 Apr 22. Expert Rev Med Devices. 2019. PMID: 30957585
-
Device profile of the EXALT Model D single-use duodenoscope for endoscopic retrograde cholangiopancreatography: overview of its safety and efficacy.Expert Rev Med Devices. 2021 May;18(5):421-427. doi: 10.1080/17434440.2021.1917990. Epub 2021 Apr 23. Expert Rev Med Devices. 2021. PMID: 33855920 Review.
-
Single-use Duodenoscope: The Cleaner Standard.J Clin Gastroenterol. 2024 Nov-Dec 01;58(10):957-962. doi: 10.1097/MCG.0000000000001994. Epub 2024 Apr 1. J Clin Gastroenterol. 2024. PMID: 38567887 Review.
Cited by
-
Uncovering the spread of drug-resistant bacteria through next-generation sequencing based surveillance: transmission of extended-spectrum β-lactamase-producing Enterobacterales by a contaminated duodenoscope.Antimicrob Resist Infect Control. 2024 Mar 8;13(1):31. doi: 10.1186/s13756-024-01386-5. Antimicrob Resist Infect Control. 2024. PMID: 38459544 Free PMC article.
-
Anlage 8: Anforderungen an die Hygiene bei der Aufbereitung thermolabiler Endoskope.Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2024 Dec;67(12):1410-1468. doi: 10.1007/s00103-024-03942-1. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2024. PMID: 39352496 German. No abstract available.
-
Adverse events, success, and tolerability of biliary endoscopic retrograde cholangiopancreatography with conscious sedation vs anaesthesia: a multi-centre prospective study.J Can Assoc Gastroenterol. 2025 Jan 23;8(2):63-70. doi: 10.1093/jcag/gwae061. eCollection 2025 Apr. J Can Assoc Gastroenterol. 2025. PMID: 40224576 Free PMC article.
-
Getting the Bugs Out: Disposable Duodenoscopes Have a Shallower Learning Curve but Face a Steep Climb in Usability.Dig Dis Sci. 2024 Jun;69(6):1918-1919. doi: 10.1007/s10620-024-08307-x. Epub 2024 Mar 11. Dig Dis Sci. 2024. PMID: 38466462 No abstract available.
-
Global prospective case series of ERCPs using a single-use duodenoscope.Endoscopy. 2023 Dec;55(12):1103-1114. doi: 10.1055/a-2131-7180. Epub 2023 Jul 18. Endoscopy. 2023. PMID: 37463599 Free PMC article. Clinical Trial.
References
-
- Anderson MA, Appalaneni V, Ben-Menachem T, et al. ; American Society for Gastrointestinal Endoscopy (ASGE) Standards of Practice Committee . The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest Endosc. 2013;77(2):167-174. doi:10.1016/j.gie.2012.09.029 - DOI - PubMed
-
- Murray P. Preventable tragedies: superbugs and how ineffective monitoring of medical device safety fails patients. January 13, 2016. Accessed May 22, 2022. https://www.help.senate.gov/imo/media/doc/Duodenoscope%20Investigation%2...
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

