Comparative Effectiveness of Immune Checkpoint Inhibitors vs Chemotherapy in Patients With Metastatic Colorectal Cancer With Measures of Microsatellite Instability, Mismatch Repair, or Tumor Mutational Burden
- PMID: 36689222
- PMCID: PMC9871803
- DOI: 10.1001/jamanetworkopen.2022.52244
Comparative Effectiveness of Immune Checkpoint Inhibitors vs Chemotherapy in Patients With Metastatic Colorectal Cancer With Measures of Microsatellite Instability, Mismatch Repair, or Tumor Mutational Burden
Abstract
Importance: The KEYNOTE-177 trial demonstrated that patients with metastatic colorectal cancer (MCRC) with high microsatellite instability (MSI-H) and/or mismatch repair deficiency (DMMR) have better outcomes when receiving first-line immune checkpoint inhibitors (ICIs) compared with chemotherapy. Data on performance of ICIs in patients with MCRC in standard practice settings remain limited, and direct MMR vs MSI outcome association comparisons are lacking.
Objective: To validate MSI (determined by next-generation sequencing [NGS]) as a biomarker of ICI effectiveness among patients with MCRC in standard practice settings and examine the association of MSI assessed by NGS, DMMR by immunohistochemistry, and tumor mutational burden (cutoff, 10 mutations/megabase) with ICI outcomes.
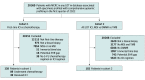
Design, setting, and participants: This comparative effectiveness research study of outcomes in prospectively defined biomarker subgroups used data from a deidentified clinicogenomic database and included patients who received Foundation Medicine testing (FoundationOne or FoundationOne CDx) during routine clinical care at approximately 280 US academic or community-based cancer clinics between March 2014 and December 2021. The population included 1 cohort of patients with MSI-H MCRC who received first-line ICIs or chemotherapy and a second cohort who received ICIs in any line of therapy (LOT) for biomarker examination.
Exposures: ICI therapy or chemotherapy assigned at physician discretion without randomization.
Main outcomes and measures: The main outcomes were time to next treatment (TTNT), progression-free survival (PFS), and overall survival (OS). Hazard ratios were adjusted for known prognostic imbalances. Comparisons of explanatory power used the likelihood ratio test.
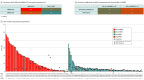
Results: A total of 138 patients (median age, 67.0 years [IQR, 56.2-74.0 years]; 73 [52.9%] female) with MSI-H MCRC received first-line ICIs or chemotherapy. A total of 182 patients (median age, 64.5 years [IQR, 55.2-72.0]; 98 [53.8%] female) received ICIs in any LOT. Patients receiving first-line ICIs vs chemotherapy had longer TTNT (median, not reached [NR] vs 7.23 months [IQR, 6.21-9.72 months]; adjusted hazard ratio [AHR], 0.17; 95% CI, 0.08-0.35; P < .001), PFS (median, 24.87 months [IQR, 19.10 months to NR] vs 5.65 months [IQR, 4.70-8.34 months]; AHR, 0.31; 95% CI, 0.18-0.52; P < .001), and OS (median, NR vs 24.1 months [IQR, 13.90 months to NR]; HR, 0.45; 95% CI, 0.23-0.88; P = .02). MSI added to DMMR better anticipated TTNT and PFS in patients receiving ICIs than DMMR alone. The same was not observed when DMMR evaluation was added to MSI.
Conclusions and relevance: In this comparative effectiveness research study, MSI assessed by NGS robustly identified patients with favorable outcomes on first-line ICIs vs chemotherapy and appeared to better anticipate ICI outcomes compared with DMMR.
Conflict of interest statement
Figures


References
-
- Diaz LA Jr, Shiu KK, Kim TW, et al. ; KEYNOTE-177 Investigators . Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022;23(5):659-670. doi:10.1016/S1470-2045(22)00197-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

