A Spontaneous Nonhuman Primate Model of Myopic Foveoschisis
- PMID: 36689233
- PMCID: PMC9896856
- DOI: 10.1167/iovs.64.1.18
A Spontaneous Nonhuman Primate Model of Myopic Foveoschisis
Abstract
Purpose: Foveoschisis involves the pathologic splitting of retinal layers at the fovea, which may occur congenitally in X-linked retinoschisis (XLRS) or as an acquired complication of myopia. XLRS is attributed to functional loss of the retinal adhesion protein retinoschisin 1 (RS1), but the pathophysiology of myopic foveoschisis is unclear due to the lack of animal models. Here, we characterized a novel nonhuman primate model of myopic foveoschisis through clinical examination and multimodal imaging followed by morphologic, cellular, and transcriptional profiling of retinal tissues and genetic analysis.
Methods: We identified a rhesus macaque with behavioral and anatomic features of myopic foveoschisis, and monitored disease progression over 14 months by fundus photography, fluorescein angiography, and optical coherence tomography (OCT). After necropsy, we evaluated anatomic and cellular changes by immunohistochemistry and transcriptomic changes using single-nuclei RNA-sequencing (snRNA-seq). Finally, we performed Sanger and whole exome sequencing with focus on the RS1 gene.
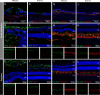
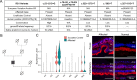
Results: Affected eyes demonstrated posterior hyaloid traction and progressive splitting of the outer plexiform layer on OCT. Immunohistochemistry showed increased GFAP expression in Müller glia and loss of ramified Iba-1+ microglia, suggesting macro- and microglial activation with minimal photoreceptor alterations. SnRNA-seq revealed gene expression changes predominantly in cones and retinal ganglion cells involving chromatin modification, suggestive of cellular stress at the fovea. No defects in the RS1 gene or its expression were detected.
Conclusions: This nonhuman primate model of foveoschisis reveals insights into how acquired myopic traction leads to phenotypically similar morphologic and cellular changes as congenital XLRS without alterations in RS1.
Conflict of interest statement
Disclosure:
Figures


References
-
- Wu PC, Chen YJ, Chen YH, et al. .. Factors associated with foveoschisis and foveal detachment without macular hole in high myopia. Eye. 2009; 23(2): 356–361. - PubMed
-
- Hahn LC, van Schooneveld MJ, Wesseling NL, et al. .. X-linked retinoschisis: novel clinical observations and genetic spectrum in 340 patients. Ophthalmology. 2022; 129(2): 191–202. - PubMed
-
- Rodríguez FJ, Rodríguez A, Mendoza-Londoño R, Tamayo ML.. X-linked retinoschisis in three females from the same family: a phenotype–genotype correlation. Retina. 2005; 25(1): 69–74. - PubMed
-
- Georgiou M, Finocchio L, Fujinami K, et al. .. X-linked retinoschisis: deep phenotyping and genetic characterization. Ophthalmology. 2022; 129(5): 542–551. - PubMed

