How does precursor RNA structure influence RNA processing and gene expression?
- PMID: 36689327
- PMCID: PMC9977717
- DOI: 10.1042/BSR20220149
How does precursor RNA structure influence RNA processing and gene expression?
Abstract
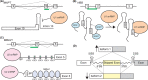
RNA is a fundamental biomolecule that has many purposes within cells. Due to its single-stranded and flexible nature, RNA naturally folds into complex and dynamic structures. Recent technological and computational advances have produced an explosion of RNA structural data. Many RNA structures have regulatory and functional properties. Studying the structure of nascent RNAs is particularly challenging due to their low abundance and long length, but their structures are important because they can influence RNA processing. Precursor RNA processing is a nexus of pathways that determines mature isoform composition and that controls gene expression. In this review, we examine what is known about human nascent RNA structure and the influence of RNA structure on processing of precursor RNAs. These known structures provide examples of how other nascent RNAs may be structured and show how novel RNA structures may influence RNA processing including splicing and polyadenylation. RNA structures can be targeted therapeutically to treat disease.
Keywords: RNA structure; alternative splicing; polyadenylation; precursor RNA; splicing; transcription.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures




Similar articles
-
Fast and furious: insights of back splicing regulation during nascent RNA synthesis.Sci China Life Sci. 2021 Jul;64(7):1050-1061. doi: 10.1007/s11427-020-1881-1. Epub 2021 Feb 9. Sci China Life Sci. 2021. PMID: 33580425 Review.
-
Revealing nascent RNA processing dynamics with nano-COP.Nat Protoc. 2021 Mar;16(3):1343-1375. doi: 10.1038/s41596-020-00469-y. Epub 2021 Jan 29. Nat Protoc. 2021. PMID: 33514943 Free PMC article.
-
In vitro polyadenylation is stimulated by the presence of an upstream intron.Genes Dev. 1990 Sep;4(9):1552-9. doi: 10.1101/gad.4.9.1552. Genes Dev. 1990. PMID: 1701407
-
Hidden regulation of herpes simplex virus 1 pre-mRNA splicing and polyadenylation by virally encoded immediate early gene ICP27.PLoS Pathog. 2019 Jun 17;15(6):e1007884. doi: 10.1371/journal.ppat.1007884. eCollection 2019 Jun. PLoS Pathog. 2019. PMID: 31206552 Free PMC article.
-
Rules of engagement: co-transcriptional recruitment of pre-mRNA processing factors.Curr Opin Cell Biol. 2005 Jun;17(3):251-6. doi: 10.1016/j.ceb.2005.04.006. Curr Opin Cell Biol. 2005. PMID: 15901493 Review.
Cited by
-
Cooperation and Competition of RNA Secondary Structure and RNA-Protein Interactions in the Regulation of Alternative Splicing.Acta Naturae. 2023 Oct-Dec;15(4):23-31. doi: 10.32607/actanaturae.26826. Acta Naturae. 2023. PMID: 38234601 Free PMC article.
-
Precursor RNA structural patterns at SF3B1 mutation sensitive cryptic 3' splice sites.bioRxiv [Preprint]. 2025 Feb 22:2025.02.19.638873. doi: 10.1101/2025.02.19.638873. bioRxiv. 2025. PMID: 40027643 Free PMC article. Preprint.
-
RNA Structure: Past, Future, and Gene Therapy Applications.Int J Mol Sci. 2024 Dec 26;26(1):110. doi: 10.3390/ijms26010110. Int J Mol Sci. 2024. PMID: 39795966 Free PMC article. Review.
-
Structural determinants of inverted Alu-mediated backsplicing revealed by -MaP and -JuMP.Nucleic Acids Res. 2025 May 10;53(9):gkaf433. doi: 10.1093/nar/gkaf433. Nucleic Acids Res. 2025. PMID: 40396491 Free PMC article.
-
TAS-seq enables subcellular single-stranded adenosine profiling by signal peptide-assisted adenosine deamination.Cell Rep Methods. 2025 Jul 21;5(7):101087. doi: 10.1016/j.crmeth.2025.101087. Epub 2025 Jun 25. Cell Rep Methods. 2025. PMID: 40570838 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

