Progesterone and prolactin levels in pregnant women living with HIV who delivered preterm and low birthweight infants: A nested case-control study
- PMID: 36689442
- PMCID: PMC9870101
- DOI: 10.1371/journal.pone.0280730
Progesterone and prolactin levels in pregnant women living with HIV who delivered preterm and low birthweight infants: A nested case-control study
Abstract
Background: Antiretroviral therapy (ART) is associated with high rates of adverse birth outcomes, including preterm birth and low birthweight. Studies suggest that progesterone and prolactin may play important intermediary roles.
Methods: We analyzed data from the Antenatal Component of the PROMISE trial, a multi-center study of pregnant women taking antiretroviral regimens (lopinavir/ritonavir-containing ART or zidovudine alone) to prevent mother-to-child HIV transmission. In a nested case-control study, we compared data from women who gave birth to preterm (<37 weeks gestation) and/or low birthweight (<2500 g) infants to matched individuals who did not. We measured serum progesterone and prolactin at 24-34 weeks gestation. We used conditional logistic regression to describe relationships between hormone levels, birth outcomes, and antiretroviral regimens.
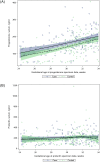
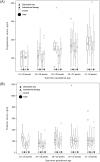
Results: 299 women and their newborns were included (146 cases, 153 controls). When compared to women receiving zidovudine alone, those on ART had higher odds of progesterone levels under the 10th percentile (adjusted odds ratio [AOR]:2.34, 95%CI:1.41-3.89) and 25th percentile (AOR:2.07, 95%CI:1.46-2.94). However, higher levels of progesterone-rather than lower levels-were associated with our composite case outcome at the 10th percentile (AOR:1.88, 95%CI:0.77-4.59) and 25th percentile (AOR:1.96, 95%CI:1.06-3.61). Associations were not observed between prolactin, antiretroviral regimen, and birth outcomes.
Conclusion: We observed lower progesterone levels among women allocated to ART regimens; however, higher progesterone levels were associated with preterm birth and/or low birthweight. While features of the study design may have contributed to these findings, they nevertheless highlight the potentially complex mechanisms underpinning adverse birth outcomes and HIV.
Copyright: © 2023 Chi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The study products in the PROMISE trial were provided free of charge by Abbott, Gilead Sciences, Boehringer Ingelheim, and GlaxoSmithKline. Funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Kesho Bora Study Group. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11(3):171–80. Epub 2011/01/18. doi: 10.1016/S1473-3099(10)70288-7 . - DOI - PubMed
-
- Joint United Nations Programme for HIV/AIDS. Start Free Stay Free AIDS Free: 2019 report. https://www.unaids.org/en/resources/documents/2019/20190722_UNAIDS_SFSFA.... Accessed on December 20, 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

