Safety and Efficacy of Ceftolozane/Tazobactam Versus Meropenem in Neonates and Children With Complicated Urinary Tract Infection, Including Pyelonephritis: A Phase 2, Randomized Clinical Trial
- PMID: 36689671
- PMCID: PMC9990597
- DOI: 10.1097/INF.0000000000003832
Safety and Efficacy of Ceftolozane/Tazobactam Versus Meropenem in Neonates and Children With Complicated Urinary Tract Infection, Including Pyelonephritis: A Phase 2, Randomized Clinical Trial
Abstract
Background: Ceftolozane/tazobactam, a cephalosporin-β-lactamase inhibitor combination, active against multidrug-resistant Gram-negative pathogens, is approved for treatment of adults with complicated urinary tract infections (cUTI). Safety and efficacy of ceftolozane/tazobactam in pediatric participants with cUTI, including pyelonephritis, were assessed.
Methods: This phase 2 study (NCT03230838) compared ceftolozane/tazobactam with meropenem for treatment of cUTI in participants from birth to <18 years of age. The primary objective was safety and tolerability. Key secondary end points included clinical cure and per-participant microbiologic response rates at end of treatment (EOT) and test of cure (TOC) visits.
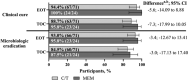
Results: The microbiologic modified intent-to-treat (mMITT) population included 95 participants (ceftolozane/tazobactam, n = 71; meropenem, n = 24). The most common diagnosis and pathogen were pyelonephritis (ceftolozane/tazobactam, 84.5%; meropenem, 79.2%) and Escherichia coli (ceftolozane/tazobactam, 74.6%; meropenem, 87.5%); 5.7% (ceftolozane/tazobactam) and 4.8% (meropenem) of E. coli isolates were extended-spectrum β-lactamase-producers. Rates of adverse events were similar between treatment groups (any: ceftolozane/tazobactam, 59.0% vs. meropenem, 60.6%; drug-related: ceftolozane/tazobactam, 14.0% vs. meropenem, 15.2%; serious: ceftolozane/tazobactam, 3.0% vs. meropenem, 6.1%). Rates of clinical cure for ceftolozane/tazobactam and meropenem at EOT were 94.4% and 100% and at TOC were 88.7% and 95.8%, respectively. Rates of microbiologic eradication for ceftolozane/tazobactam and meropenem at EOT were 93.0% and 95.8%, and at TOC were 84.5% and 87.5%, respectively.
Conclusions: Ceftolozane/tazobactam had a favorable safety profile in pediatric participants with cUTI; rates of clinical cure and microbiologic eradication were high and similar to meropenem. Ceftolozane/tazobactam is a safe and effective new treatment option for children with cUTI, especially due to antibacterial-resistant Gram-negative pathogens.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Figures

References
-
- World Health Organization. WHO publishes list of bacteria for which new antibiotics are urgently needed. Available at: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-.... Accessed February 28, 2022.
-
- FDA. Complicated urinary tract infections: developing drugs for treatment. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents.... Accessed January 11, 2023..
-
- Koçak M, Büyükkaragöz B, Çelebi Tayfur A, et al. Causative pathogens and antibiotic resistance in children hospitalized for urinary tract infection. Pediatr Int. 2016;58:467–471. - PubMed
-
- Lutter SA, Currie ML, Mitz LB, et al. Antibiotic resistance patterns in children hospitalized for urinary tract infections. Arch Pediatr Adolesc Med. 2005;159:924–928. - PubMed

