Tarlatamab, a First-in-Class DLL3-Targeted Bispecific T-Cell Engager, in Recurrent Small-Cell Lung Cancer: An Open-Label, Phase I Study
- PMID: 36689692
- PMCID: PMC10414718
- DOI: 10.1200/JCO.22.02823
Tarlatamab, a First-in-Class DLL3-Targeted Bispecific T-Cell Engager, in Recurrent Small-Cell Lung Cancer: An Open-Label, Phase I Study
Abstract
Purpose: Small-cell lung cancer (SCLC) is an aggressive malignancy with limited treatments. Delta-like ligand 3 (DLL3) is aberrantly expressed in most SCLC. Tarlatamab (AMG 757), a bispecific T-cell engager molecule, binds both DLL3 and CD3 leading to T-cellb-mediated tumor lysis. Herein, we report phase I results of tarlatamab in patients with SCLC.
Patients and methods: This study evaluated tarlatamab in patients with relapsed/refractory SCLC. The primary end point was safety. Secondary end points included antitumor activity by modified RECIST 1.1, overall survival, and pharmacokinetics.
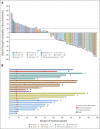
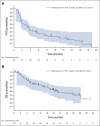
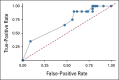
Results: By July 19, 2022, 107 patients received tarlatamab in dose exploration (0.003 to 100 mg; n = 73) and expansion (100 mg; n = 34) cohorts. Median prior lines of anticancer therapy were 2 (range, 1-6); 49.5% received antiprogrammed death-1/programmed death ligand-1 therapy. Any-grade treatment-related adverse events occurred in 97 patients (90.7%) and grade b % 3 in 33 patients (30.8%). One patient (1%) had grade 5 pneumonitis. Cytokine release syndrome was the most common treatment-related adverse event, occurring in 56 patients (52%) including grade 3 in one patient (1%). Maximum tolerated dose was not reached. Objective response rate was 23.4% (95% CI, 15.7 to 32.5) including two complete and 23 partial responses. The median duration of response was 12.3 months (95% CI, 6.6 to 14.9). The disease control rate was 51.4% (95% CI, 41.5 to 61.2). The median progression-free survival and overall survival were 3.7 months (95% CI, 2.1 to 5.4) and 13.2 months (95% CI, 10.5 to not reached), respectively. Exploratory analysis suggests that selecting for increased DLL3 expression can result in increased clinical benefit.
Conclusion: In patients with heavily pretreated SCLC, tarlatamab demonstrated manageable safety with encouraging response durability. Further evaluation of this promising molecule is ongoing.
Trial registration: ClinicalTrials.gov NCT03319940.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
Comment in
-
Tarlatamab: New Star on the Horizon for Small-Cell Lung Cancer?J Clin Oncol. 2023 Jun 1;41(16):2877-2880. doi: 10.1200/JCO.23.00148. Epub 2023 Apr 25. J Clin Oncol. 2023. PMID: 37098228 Free PMC article. No abstract available.
-
Tarlatamab: the promising immunotherapy on its way from the lab to the clinic.Transl Lung Cancer Res. 2023 Jun 30;12(6):1355-1357. doi: 10.21037/tlcr-23-115. Epub 2023 Mar 20. Transl Lung Cancer Res. 2023. PMID: 37425422 Free PMC article. No abstract available.
-
Tarlatamab: a potential new option for recurrent small cell lung cancer.Transl Lung Cancer Res. 2023 Jul 31;12(7):1628-1630. doi: 10.21037/tlcr-23-215. Epub 2023 Jun 5. Transl Lung Cancer Res. 2023. PMID: 37577302 Free PMC article. No abstract available.
References
-
- von Pawel J, Schiller JH, Shepherd FA, et al. : Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 17:658-667, 1999 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

