TNFR1 links TNF exocytosis to TNF production in allergen-activated RBL-2H3 cells
- PMID: 36690134
- PMCID: PMC10122983
- DOI: 10.1016/j.cellsig.2023.110607
TNFR1 links TNF exocytosis to TNF production in allergen-activated RBL-2H3 cells
Abstract
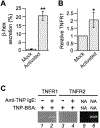
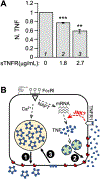
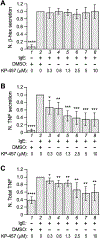
We previously reported that the maximal production of Tumor Necrosis Factor (TNF or TNFα) in antigen-activated RBL-2H3 cells (a tumor analog of mucosal mast cells) requires Munc13-4, a regulator of exocytic fusion. In this study, we investigated the involvement of various fusion catalysts in TNF production. We observed a strong correlation between the total TNF level and TNF exocytosis in RBL-2H3 cells. RT-qPCR shows that TNFR1 (TNF receptor 1) is the sole TNFR expressed in these cells, and that its transcription is upregulated upon allergen-mediated activation. Importantly, the addition of soluble TNFR1 inhibits antigen-elicited TNF production in a dosage-dependent fashion. Likewise, TNF production is diminished in the presence of TACE (TNFα Converting Enzyme) inhibitor KP-457, which prevents the generation of soluble TNF (sTNF). Together, these findings indicate that sTNF and TNFR1 function as autocrine agent and receptor respectively at the mast cell surface to boost TNF proliferation during allergic inflammation.
Keywords: Allergy; Autocrine signaling; Degranulation; Inflammation; Mast cell; Munc18.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing financial interests.
Figures

Similar articles
-
TNF Production in Activated RBL-2H3 Cells Requires Munc13-4.Inflammation. 2020 Apr;43(2):744-751. doi: 10.1007/s10753-019-01161-4. Inflammation. 2020. PMID: 31897916 Free PMC article.
-
Exocytic machineries differentially control mediator release from allergen-triggered RBL-2H3 cells.Inflamm Res. 2023 Mar;72(3):639-649. doi: 10.1007/s00011-023-01698-z. Epub 2023 Feb 1. Inflamm Res. 2023. PMID: 36725743
-
A novel eremophilane lactone inhibits FcεRI-dependent release of pro-inflammatory mediators: structure-dependent bioactivity.Inflamm Res. 2016 Apr;65(4):303-11. doi: 10.1007/s00011-016-0917-2. Epub 2016 Jan 20. Inflamm Res. 2016. PMID: 26791350
-
Gene expression and production of tumour necrosis factor by a rat basophilic leukaemia cell line (RBL-2H3) with IgE receptor triggering.Immunology. 1990 May;70(1):88-93. Immunology. 1990. PMID: 2141321 Free PMC article.
-
RBL cells as models for in vitro studies of mast cells and basophils.Immunol Rev. 2018 Mar;282(1):47-57. doi: 10.1111/imr.12628. Immunol Rev. 2018. PMID: 29431208 Review.
Cited by
-
Aluminum Disrupts Motor Function, Cholinergic System, Redox Balance, Pro-Apoptotic and Pro-Inflammatory Pathways in Nauphoeta cinerea Model.Neurochem Res. 2025 Jun 27;50(4):217. doi: 10.1007/s11064-025-04461-4. Neurochem Res. 2025. PMID: 40576726
-
An Autocrine Regulator Loop Involving Tumor Necrosis Factor and Chemokine (C-C motif) Ligand-2 Is Activated by Transforming Growth Factor-β in Rat Basophilic Leukemia-2H3 Mast Cells.Int J Mol Sci. 2025 Apr 30;26(9):4263. doi: 10.3390/ijms26094263. Int J Mol Sci. 2025. PMID: 40362499 Free PMC article.
References
-
- Schulz R and Heusch G, Tumor necrosis factor-alpha and its receptors 1 and 2: Yin and Yang in myocardial infarction? Circulation, 2009. 119(10): p. 1355–7. - PubMed
-
- Aoki R, et al., Mast cells play a key role in host defense against herpes simplex virus infection through TNF-alpha and IL-6 production. J Invest Dermatol, 2013. 133(9): p. 2170–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

