Tumefactive Demyelination in MOG Ab-Associated Disease, Multiple Sclerosis, and AQP-4-IgG-Positive Neuromyelitis Optica Spectrum Disorder
- PMID: 36690455
- PMCID: PMC10065219
- DOI: 10.1212/WNL.0000000000206820
Tumefactive Demyelination in MOG Ab-Associated Disease, Multiple Sclerosis, and AQP-4-IgG-Positive Neuromyelitis Optica Spectrum Disorder
Abstract
Background and objectives: Studies on tumefactive brain lesions in myelin oligodendrocyte glycoprotein-immunoglobulin G (IgG)-associated disease (MOGAD) are lacking. We sought to characterize the frequency clinical, laboratory, and MRI features of these lesions in MOGAD and compare them with those in multiple sclerosis (MS) and aquaporin-4-IgG-positive neuromyelitis optica spectrum disorder (AQP4+NMOSD).
Methods: We retrospectively searched 194 patients with MOGAD and 359 patients with AQP4+NMOSD with clinical/MRI details available from the Mayo Clinic databases and included those with ≥1 tumefactive brain lesion (maximum transverse diameter ≥2 cm) on MRI. Patients with tumefactive MS were identified using the Mayo Clinic medical record linkage system. Binary multivariable stepwise logistic regression identified independent predictors of MOGAD diagnosis; Cox proportional regression models were used to assess the risk of relapsing disease and gait aid in patients with tumefactive MOGAD vs those with nontumefactive MOGAD.
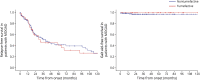
Results: We included 108 patients with tumefactive demyelination (MOGAD = 43; AQP4+NMOSD = 16; and MS = 49). Tumefactive lesions were more frequent among those with MOGAD (43/194 [22%]) than among those with AQP4+NMOSD (16/359 [5%], p < 0.001). Risk of relapse and need for gait aid were similar in tumefactive and nontumefactive MOGAD. Clinical features more frequent in MOGAD than in MS included headache (18/43 [42%] vs 10/49 [20%]; p = 0.03) and somnolence (12/43 [28%] vs 2/49 [4%]; p = 0.003), the latter also more frequent than in AQP4+NMOSD (0/16 [0%]; p = 0.02). The presence of peripheral T2-hypointense rim, T1-hypointensity, diffusion restriction (particularly an arc pattern), ring enhancement, and Baló-like or cystic appearance favored MS over MOGAD (p ≤ 0.001). MRI features were broadly similar in MOGAD and AQP4+NMOSD, except for more frequent diffusion restriction in AQP4+NMOSD (10/15 [67%]) than in MOGAD (11/42 [26%], p = 0.005). CSF analysis revealed less frequent positive oligoclonal bands in MOGAD (2/37 [5%]) than in MS (30/43 [70%], p < 0.001) and higher median white cell count in MOGAD than in MS (33 vs 6 cells/μL, p < 0.001). At baseline, independent predictors of MOGAD diagnosis were the presence of somnolence/headache, absence of T2-hypointense rim, lack of T1-hypointensity, and no diffusion restriction (Nagelkerke R 2 = 0.67). Tumefactive lesion resolution was more common in MOGAD than in MS or AQP4+NMOSD and improved model performance.
Discussion: Tumefactive lesions are frequent in MOGAD but not associated with a worse prognosis. The clinical, MRI, and CSF attributes of tumefactive MOGAD differ from those of tumefactive MS and are more similar to those of tumefactive AQP4+NMOSD with the exception of lesion resolution, which favors MOGAD.
© 2023 American Academy of Neurology.
Conflict of interest statement
L. Cacciaguerra received speaker and consultant honoraria from ACCMED, Roche, BMS Celgene, and Sanofi. P. Morris reports no disclosures. W.O. Tobin has received research funding from Mallinckrodt Inc., the Mayo Clinic Center for Multiple Sclerosis and Autoimmune Neurology, and the NIH (grant R01NS113803 and R01NS121928) outside the submitted work; he has received honoraria from Neurology Live and has coedited
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
