Colitis in a transgenic mouse model of autoimmune uveitis may be induced by neoantigen presentation in the bowel
- PMID: 36690619
- PMCID: PMC9870966
- DOI: 10.1038/s41598-022-27018-9
Colitis in a transgenic mouse model of autoimmune uveitis may be induced by neoantigen presentation in the bowel
Abstract
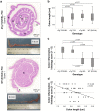
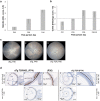
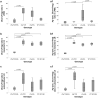
Undifferentiated uveitis (intraocular inflammation, IOI) is an idiopathic sight-threatening, presumed autoimmune disease, accountable for ~ 10% of all blindness in the developed world. We have investigated the association of uveitis with inflammatory bowel disease (IBD) using a mouse model of spontaneous experimental autoimmune uveoretinitis (EAU). Mice expressing the transgene (Tg) hen egg lysozyme (HEL) in the retina crossed with 3A9 mice expressing a transgenic HEL-specific TCR spontaneously develop uveoretinitis at post-partum day (P)20/21. Double transgenic (dTg TCR/HEL) mice also spontaneously develop clinical signs of colitis at ~ P30 with diarrhoea, bowel shortening, oedema and lamina propria (LP) inflammatory cell infiltration. Single (s)Tg TCR (3A9) mice also show increased histological LP cell infiltration but no bowel shortening and diarrhoea. dTg TCR/HEL mice are profoundly lymphopenic at weaning. In addition, dTg TCR/HEL mice contain myeloid cells which express MHC Class II-HEL peptide complexes (MHCII-HEL), not only in the inflamed retina but also in the colon and have the potential for antigen presentation. In this model the lymphopenia and reduction in the absolute Treg numbers in dTg TCR/HEL mice is sufficient to initiate eye disease. We suggest that cell-associated antigen released from the inflamed eye can activate colonic HEL-specific T cells which, in a microbial micro-environment, not only cause colitis but feedback to amplify IOI.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. RC is a founder of MIROBio Ltd.
Figures



Similar articles
-
Treatment With FoxP3+ Antigen-Experienced T Regulatory Cells Arrests Progressive Retinal Damage in a Spontaneous Model of Uveitis.Front Immunol. 2020 Sep 4;11:2071. doi: 10.3389/fimmu.2020.02071. eCollection 2020. Front Immunol. 2020. PMID: 33013877 Free PMC article.
-
Targeted delivery of autoantigen to dendritic cells prevents development of spontaneous uveitis.Front Immunol. 2023 Sep 1;14:1227633. doi: 10.3389/fimmu.2023.1227633. eCollection 2023. Front Immunol. 2023. PMID: 37727784 Free PMC article.
-
The level of peptide-MHC complex determines the susceptibility to autoimmune diabetes: studies in HEL transgenic mice.Eur J Immunol. 2001 Dec;31(12):3453-9. doi: 10.1002/1521-4141(200112)31:12<3453::aid-immu3453>3.0.co;2-h. Eur J Immunol. 2001. PMID: 11745364
-
Maturation and function of mouse T-cells with a transgenic TCR positively selected by highly disparate xenogeneic porcine MHC.Cell Mol Biol (Noisy-le-grand). 2001 Feb;47(1):217-28. Cell Mol Biol (Noisy-le-grand). 2001. PMID: 11292257
-
Antigen presentation in uveitis.Eye (Lond). 1997;11 ( Pt 2):176-82. doi: 10.1038/eye.1997.48. Eye (Lond). 1997. PMID: 9349409 Review.
Cited by
-
Discovery of Two Novel Immunoepitopes and Development of a Peptide-based Sarcoidosis Immunoassay.Am J Respir Crit Care Med. 2024 Oct 1;210(7):908-918. doi: 10.1164/rccm.202306-1054OC. Am J Respir Crit Care Med. 2024. PMID: 38385694
-
Whey Protein Hydrolysate Exerts Anti-Inflammatory Effects to Alleviate Dextran Sodium Sulfate (DSS)-Induced Colitis via Microbiome Restoration.Nutrients. 2023 Oct 17;15(20):4393. doi: 10.3390/nu15204393. Nutrients. 2023. PMID: 37892468 Free PMC article.
References
-
- Scherer, H. U., van der Woude, D. & Toes, R. E. From risk to chronicity: Evolution of autoreactive B cell and antibody responses in rheumatoid arthritis. Nat. Rev. Rheumatol. 1–13 (2022). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

