The kinetics of carbon pair formation in silicon prohibits reaching thermal equilibrium
- PMID: 36690635
- PMCID: PMC9870972
- DOI: 10.1038/s41467-023-36090-2
The kinetics of carbon pair formation in silicon prohibits reaching thermal equilibrium
Abstract
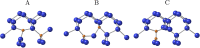
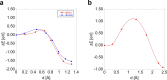
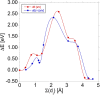
Thermal equilibrium is reached when the system assumes its lowest energy. This can be hindered by kinetic reasons; however, it is a general assumption that the ground state can be eventually reached. Here, we show that this is not always necessarily the case. Carbon pairs in silicon have at least three different configurations, one of them (B-configuration) is the G photoluminescence centre. Experiments revealed a bistable nature with the A-configuration. Electronic structure calculations predicted that the C-configuration is the real ground state; however, no experimental evidence was found for its existence. Our calculations show that the formation of the A- and B-configurations is strongly favoured over the most stable C-configuration which cannot be realized in a detectable amount before the pair dissociates. Our results demonstrate that automatized search for complex defects consisting of only the thermodynamically most stable configurations may overlook key candidates for quantum technology applications.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Gao, F., Posselt, M., Belko, V., Zhang, Y. & Weber, W. J. Structures and energetics of defects: a comparative study of 3C- and 4H-SiC, Nucl. Instrum. Methods Phys. Res. B: Beam Interact. Mater. Atoms218, 74 (2004).
-
- Gharaibeh, M., Estreicher, S. K. & Fedders, P. A. Molecular-dynamics studies of self-interstitial aggregates in Si. Phys. B Condensed Matter273–274, 532 (1999).
-
- Davidsson, J., Ivády, V., Armiento, R. & Abrikosov, I. A. ADAQ: automatic workflows for magneto-optical properties of point defects in semiconductors. Comput. Phys. Commun.269, 108091 (2021).
-
- Bertoldo F, Ali S, Manti S, Thygesen KS. Quantum point defects in 2D materials - the QPOD database. npj Comput. Mater. 2022;8:56. doi: 10.1038/s41524-022-00730-w. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

