Does treatment with autophagy-enhancers and/or ROS-scavengers alleviate behavioral and neurochemical consequences of low-dose rotenone-induced mild mitochondrial dysfunction in mice?
- PMID: 36690794
- PMCID: PMC10208973
- DOI: 10.1038/s41380-023-01955-x
Does treatment with autophagy-enhancers and/or ROS-scavengers alleviate behavioral and neurochemical consequences of low-dose rotenone-induced mild mitochondrial dysfunction in mice?
Abstract
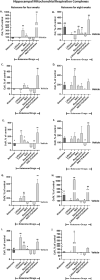
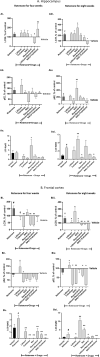
Bipolar-disorder's pathophysiology and the mechanism by which medications exert their beneficial effect is yet unknown, but others' and our data implicate patients' brain mitochondrial-dysfunction and its amendment by mood-stabilizers. We recently designed a novel mouse bipolar-disorder-like model using chronic administration of a low-dose of the oxidative-phosphorylation complex I inhibitor, rotenone. Four and eight weeks rotenone treatment induced manic- and depressive-like behavior, respectively, accompanied by mood-related neurochemical changes. Here we aimed to investigate whether each of the autophagy-enhancers lithium (a mood-stabilizer), trehalose and resveratrol and/or each of the reactive oxygen species (ROS)-scavengers, resveratrol and N-acetylcystein and/or the combinations lithium+resveratrol or trehalose+N-acetylcystein, can ameliorate behavioral and neurochemical consequences of neuronal mild mitochondrial-dysfunction. We observed that lithium, trehalose and N-acetylcystein reversed rotenone-induced manic-like behavior as well as deviations in protein levels of mitochondrial complexes and the autophagy marker LC3-II. This raises the possibility that mild mitochondrial-dysfunction accompanied by impaired autophagy and a very mild increase in ROS levels are related to predisposition to manic-like behavior. On the other hand, although, as expected, most of the drugs tested eliminated the eight weeks rotenone-induced increase in protein levels of all hippocampal mitochondrial complexes, only lithium ubiquitously ameliorated the depressive-like behaviors. We cautiously deduce that aberrant autophagy and/or elevated ROS levels are not involved in predisposition to the depressive phase of bipolar-like behavior. Rather, that amending the depressive-like characteristics requires different mitochondria-related interventions. The latter might be antagonizing N-methyl-D-aspartate receptors (NMDARs), thus protecting from disruption of mitochondrial calcium homeostasis and its detrimental consequences. In conclusion, our findings suggest that by-and-large, among the autophagy-enhancers and ROS-scavengers tested, lithium is the most effective in counteracting rotenone-induced changes. Trehalose and N-acetylcystein may also be effective in attenuating manic-like behavior.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Do Autophagy Enhancers/ROS Scavengers Alleviate Consequences of Mild Mitochondrial Dysfunction Induced in Neuronal-Derived Cells?Int J Mol Sci. 2021 May 27;22(11):5753. doi: 10.3390/ijms22115753. Int J Mol Sci. 2021. PMID: 34072255 Free PMC article.
-
Using mitochondrial respiration inhibitors to design a novel model of bipolar disorder-like phenotype with construct, face and predictive validity.Transl Psychiatry. 2021 Feb 12;11(1):123. doi: 10.1038/s41398-021-01215-y. Transl Psychiatry. 2021. PMID: 33579900 Free PMC article.
-
Resveratrol provides neuroprotective effects through modulation of mitochondrial dynamics and ERK1/2 regulated autophagy.Free Radic Res. 2018 Dec;52(11-12):1371-1386. doi: 10.1080/10715762.2018.1489128. Free Radic Res. 2018. PMID: 30693838
-
[Antipsychotics in bipolar disorders].Encephale. 2004 Sep-Oct;30(5):417-24. doi: 10.1016/s0013-7006(04)95456-5. Encephale. 2004. PMID: 15627046 Review. French.
-
Important role of mitochondria and the effect of mood stabilizers on mitochondrial function.Physiol Res. 2019 Nov 22;68(Suppl 1):S3-S15. doi: 10.33549/physiolres.934324. Physiol Res. 2019. PMID: 31755286 Review.
Cited by
-
The Protective Effect of Lithium Against Rotenone may be Evolutionarily Conserved: Evidence from Eisenia fetida, a Primitive Animal with a Ganglionic Brain.Neurochem Res. 2023 Dec;48(12):3538-3559. doi: 10.1007/s11064-023-04001-y. Epub 2023 Aug 1. Neurochem Res. 2023. PMID: 37526866
-
Mitochondria in depression: The dysfunction of mitochondrial energy metabolism and quality control systems.CNS Neurosci Ther. 2024 Feb;30(2):e14576. doi: 10.1111/cns.14576. CNS Neurosci Ther. 2024. PMID: 38334212 Free PMC article. Review.
-
Non-canonical pathways in the pathophysiology and therapeutics of bipolar disorder.Front Neurosci. 2023 Aug 1;17:1228455. doi: 10.3389/fnins.2023.1228455. eCollection 2023. Front Neurosci. 2023. PMID: 37592949 Free PMC article. Review.
-
The Association Among Bipolar Disorder, Mitochondrial Dysfunction, and Reactive Oxygen Species.Biomolecules. 2025 Mar 6;15(3):383. doi: 10.3390/biom15030383. Biomolecules. 2025. PMID: 40149919 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

