Clinical outcomes in patients relapsed/refractory after ≥2 prior lines of therapy for follicular lymphoma: a systematic literature review and meta-analysis
- PMID: 36690960
- PMCID: PMC9869623
- DOI: 10.1186/s12885-023-10546-6
Clinical outcomes in patients relapsed/refractory after ≥2 prior lines of therapy for follicular lymphoma: a systematic literature review and meta-analysis
Abstract
Background: Patients with follicular lymphoma (FL) can have high response rates to early lines of treatment. However, among FL patients relapsed/refractory (r/r) after ≥2 prior lines of therapy (LOT), remission tends to be shorter and there is limited treatment guidance. This study sought to evaluate the clinical outcomes for r/r FL after ≥2 prior LOT identified through systematic literature review.
Methods: Eligible studies included comparative or non-comparative interventional or observational studies of systemic therapies among adults with FL r/r after ≥2 prior LOT published prior to 31st May 2021. Prior LOT must have included an anti-CD20 monoclonal antibody and an alkylating agent, in combination or separately. Overall response rate (ORR) and complete response (CR) were estimated using inverse-variance weighting with Freeman-Tukey double-arcsine transformations. Kaplan-Meier (KM) curves for progression-free survival (PFS) and overall survival (OS) estimated by reconstructing digitized curves using the Guyot algorithm, and survival analyses were conducted, stratified by ≥2 prior LOT and ≥ 3 prior LOT groups (as defined in the source material). Restricting the analyses to the observational cohorts was investigated as a sensitivity analysis.
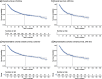
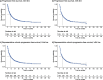
Results: The analysis-set included 20 studies published between 2014 and 2021. Studies were primarily US and/or European based, with the few exceptions using treatments approved in US/Europe. The estimated ORR was 58.47% (95% confidence interval [CI]: 51.13-65.62) and proportion of patients with CR was 19.63% (95% CI: 15.02-24.68). The median OS among those ≥2 prior LOT was 56.57 months (95% CI: 47.8-68.78) and median PFS was 9.78 months (95% CI: 9.01-10.63). The 24-month OS decreased from 66.50% in the ≥2 prior LOT group to 59.51% in the ≥3 prior LOT group, with a similar trend in PFS at 24-month (28.42% vs 24.13%).
Conclusions: This study found that few r/r FL patients with ≥2 prior LOT achieve CR, and despite some benefit, approximately 1/3 of treated patients die within 24 months. The shorter median PFS with increasing prior LOT suggest treatment durability is suboptimal in later LOT. These findings indicate that patients are underserved by treatments currently available in the US and Europe.
Keywords: Clinical outcomes; Meta-analysis; Relapsed/refractory follicular lymphoma; Systematic literature review.
© 2023. The Author(s).
Conflict of interest statement
SK: Employment or leadership position: RainCity Analytics; Research funding: RainCity Analytics has received funds from for-profit healthcare companies for research. BK: Consulting: Abbvie, Acerta, Astra Zeneca, ADCT, Celgene, Genentech, Juno, Kite, Morphosys, BeiGene, Pharmacyclics, Janssen, Genmab, Incyte, MEI; Research Funding: Genentech, ADCT, Acerta, Celgene, BeiGene. AW: Employment or leadership position - Kite, A Gilead company; Stock ownership - Kite, A Gilead company. BG: Employment or leadership position: IQVIA. EHLO: Employment or leadership position: RainCity Analytics. AS: Employment or leadership position: Atara Biotherapeutics, Kite Pharma; Stock ownership: Atara Biotherapeutics, Gilead. GB: Employment or leadership position - Kite, A Gilead company; Stock ownership - Kite, A Gilead company. JTS: Employment or leadership position: Kite, A Gilead Company, Stock ownership: Gilead Sciences. AP: Employment or leadership position - Kite, A Gilead company; Stock ownership - Kite, A Gilead company.
Figures

References
-
- International Agency for Research on Cancer. Non-Hodgkin Lymphoma. 2020. https://gco.iarc.fr/today/data/factsheets/cancers/34-Non-hodgkin-lymphom...
-
- Casulo C, Byrtek M, Dawson KL, et al. Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study. J Clin Oncol. 2015;33(23):2516–2522. doi: 10.1200/jco.2014.59.7534. - DOI - PMC - PubMed
-
- Leukemia and Lymphoma Society. NHL subtypes https://www.lls.org/lymphoma/non-hodgkin-lymphoma/diagnosis/nhl-subtypes
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

