The global prevalence of parasites in non-biting flies as vectors: a systematic review and meta-analysis
- PMID: 36691084
- PMCID: PMC9872427
- DOI: 10.1186/s13071-023-05650-2
The global prevalence of parasites in non-biting flies as vectors: a systematic review and meta-analysis
Abstract
Background: Non-biting flies such as the house fly (Musca domestica), the Australian sheep blowfly (Lucilia cuprina) and the oriental latrine fly (Chrysomya megacephala) may carry many parasites. In the present study, we performed a systematic overview of the different species of parasites carried by non-biting flies, as well as of isolation methods, different geographical distribution, seasonality and risk assessment.
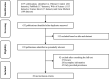
Methods: A meta-analysis was carried out with the aim to review the global prevalence of parasite transmission in non-biting flies. A total sample size of 28,718 non-biting flies reported in studies worldwide satisfied the predetermined selection criteria and was included in the quantitative analysis.
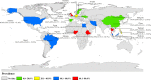
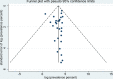
Results: The global prevalence of parasites in non-biting flies was 42.5% (95% confidence interval [CI] 31.9-53.2%; n = 15,888/28,718), with the highest prevalence found for non-biting flies in Africa (58.3%; 95% CI 47.4-69.3%; n = 9144/13,366). A total of 43% (95% CI 32.1-54.4%; n = 7234/15,282) of house flies (M. domestica), the fly species considered to be the most closely associated with humans and animals, were found with parasites. The prevalence of parasites in the intestine of non-biting flies was 37.1% (95% CI 22.7-51.5%; n = 1045/3817), which was significantly higher than the prevalence of parasites isolated from the body surface (35.1%; 95% CI 20.8-49.4%; n = 1199/3649; P < 0.01). Of the 27 reported parasites, a total of 20 known zoonotic parasites were identified, with an infection rate of 38.1% (95% CI 28.2-48.0%; n = 13,572/28,494).
Conclusions: This study provides a theoretical basis for the public health and ecological significance of parasites transmitted by non-biting flies.
Keywords: Meta-analysis; Non-biting flies; Parasites; Vectors.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures








Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
Cited by
-
Study on genetic characteristics of Cryptosporidium isolates and first report of C. parvum IIdA24G2 subtype in dairy cattle in China.Parasitol Res. 2024 Jan 2;123(1):81. doi: 10.1007/s00436-023-08107-8. Parasitol Res. 2024. PMID: 38165486
-
Simultaneous detection of seven bacterial pathogens transmitted by flies using the reverse line blot hybridization assay.Parasit Vectors. 2024 Feb 22;17(1):82. doi: 10.1186/s13071-024-06170-3. Parasit Vectors. 2024. PMID: 38389104 Free PMC article.
-
Sympatric non-biting flies serve as potential vectors of zoonotic protozoan parasites on pig farms in China.Parasit Vectors. 2025 Feb 18;18(1):59. doi: 10.1186/s13071-025-06686-2. Parasit Vectors. 2025. PMID: 39966891 Free PMC article.
-
Unveiling the hidden dangers: enteropathogens carried by flies in Pudong New Area.BMC Infect Dis. 2024 Jun 7;24(1):569. doi: 10.1186/s12879-024-09448-0. BMC Infect Dis. 2024. PMID: 38849747 Free PMC article.
-
Blowflies (Diptera: Calliphoridae) as potential mechanical vectors of the protozoan cyst and helminthic eggs in Kashmir Himalaya, India.J Parasit Dis. 2024 Jun;48(2):283-288. doi: 10.1007/s12639-024-01663-5. Epub 2024 Apr 6. J Parasit Dis. 2024. PMID: 38840884 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

