Better use of inhaled medication in asthma and COPD through training, preparation and counselling: the On TRACk study protocol for a cluster randomised controlled trial
- PMID: 36691116
- PMCID: PMC9454022
- DOI: 10.1136/bmjopen-2022-061266
Better use of inhaled medication in asthma and COPD through training, preparation and counselling: the On TRACk study protocol for a cluster randomised controlled trial
Abstract
Introduction: About 70% of patients with asthma and/or chronic obstructive pulmonary disease (COPD) use their inhaled medication incorrectly, leading to reduced disease control, higher healthcare use and costs. Adequate guidance from the pharmacy team from first dispense onwards can benefit patients in the long run. We propose an intervention ('On TRACk') to improve medication adherence and inhaler technique of adult patients with asthma and/or COPD. This intervention focuses on training pharmacy technicians (PTs) in patient-centred communication and inhalation instruction skills. In addition, patients are actively involved in refill consultations at the pharmacy. The aim of this study is to improve inhaler technique and better inhaled medication adherence among patients with asthma and/or COPD. This paper describes the study protocol.
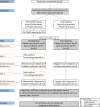
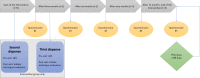
Methods and analysis: A cluster randomised controlled trial (RCT) with an intervention and control group of 15 pharmacies each will be conducted. Per intervention pharmacy, two PTs will be trained online. Each PT will include five patients who will prepare their second and third dispense counselling sessions by selecting three topics they wish to discuss. Pharmacies in the control cluster provide usual care. In total, 300 patients (150 per group) will be included. Up to 12 months after inclusion, patients complete 3-monthly follow-up questionnaires. Both a process evaluation and a cost-effectiveness analysis will be performed alongside the trial. Trial effectiveness on the patient level will be evaluated after the 12-month follow-up period.Patient data will be collected through questionnaires and pharmacy refill data. Patients' inhaler technique will be visually assessed by PTs. Semistructured interviews with PTs and patients will be conducted regarding implementation and fidelity. Direct and indirect health costs will be collected to assess cost-effectiveness.The primary outcome is adherence to inhalation maintenance medication measured with pharmacy refill data. Secondary outcomes are inhaler technique, persistence, patients' attitudes towards medication, self-efficacy in medication use and communication with their PTs.
Ethics and dissemination: The study was approved by the Vrije Universiteit Amsterdam Ethics Committee (number: 2020.358). Results will be presented at (inter)national conferences and published in peer-reviewed journals. If proven to be (cost-)effective, the intervention should be considered for reimbursement and implementation in Dutch community pharmacies.
Trial registration number: NL9750.
Keywords: Asthma; Chronic airways disease; MEDICAL EDUCATION & TRAINING; RESPIRATORY MEDICINE (see Thoracic Medicine).
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors declare not to have any competing interests that are related to this study.
Figures
Similar articles
-
Inhaler technique education in elderly patients with asthma or COPD: impact on disease exacerbations-a protocol for a single-blinded randomised controlled trial.BMJ Open. 2019 Jan 28;9(1):e022685. doi: 10.1136/bmjopen-2018-022685. BMJ Open. 2019. PMID: 30696670 Free PMC article.
-
The effect of providing feedback on inhaler technique and adherence from an electronic audio recording device, INCA®, in a community pharmacy setting: study protocol for a randomised controlled trial.Trials. 2016 May 4;17(1):226. doi: 10.1186/s13063-016-1362-9. Trials. 2016. PMID: 27142873 Free PMC article. Clinical Trial.
-
Effectiveness of a pharmacist-driven intervention in COPD (EPIC): study protocol for a randomized controlled trial.Trials. 2016 Oct 13;17(1):502. doi: 10.1186/s13063-016-1623-7. Trials. 2016. PMID: 27737686 Free PMC article. Clinical Trial.
-
Effect of pharmacist-led interventions on medication adherence and inhalation technique in adult patients with asthma or COPD: A systematic review and meta-analysis.J Clin Pharm Ther. 2020 Oct;45(5):904-917. doi: 10.1111/jcpt.13126. Epub 2020 Feb 27. J Clin Pharm Ther. 2020. PMID: 32107837
-
Impact of Single Combination Inhaler versus Multiple Inhalers to Deliver the Same Medications for Patients with Asthma or COPD: A Systematic Literature Review.Int J Chron Obstruct Pulmon Dis. 2020 Feb 26;15:417-438. doi: 10.2147/COPD.S234823. eCollection 2020. Int J Chron Obstruct Pulmon Dis. 2020. PMID: 32161454 Free PMC article.
Cited by
-
Methods to assess COPD medications adherence in healthcare databases: a systematic review.Eur Respir Rev. 2023 Sep 27;32(169):230103. doi: 10.1183/16000617.0103-2023. Print 2023 Sep 30. Eur Respir Rev. 2023. PMID: 37758274 Free PMC article.
References
-
- Lewis A, Torvinen S, Dekhuijzen PNR, et al. . The economic burden of asthma and chronic obstructive pulmonary disease and the impact of poor inhalation technique with commonly prescribed dry powder inhalers in three European countries. BMC Health Serv Res 2016;16:1–12. 10.1186/s12913-016-1482-7 - DOI - PMC - PubMed
-
- Asthma GIf. Global strategy for asthma management and prevention, 2021.
-
- GOLD, G.I.f.C.O.L.D . Global strategy for prevention, Diagnonis and management of COPD, 2022.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical