Quality of reporting of randomised controlled trials of artificial intelligence in healthcare: a systematic review
- PMID: 36691151
- PMCID: PMC9445816
- DOI: 10.1136/bmjopen-2022-061519
Quality of reporting of randomised controlled trials of artificial intelligence in healthcare: a systematic review
Abstract
Objectives: The aim of this study was to evaluate the quality of reporting of randomised controlled trials (RCTs) of artificial intelligence (AI) in healthcare against Consolidated Standards of Reporting Trials-AI (CONSORT-AI) guidelines.
Design: Systematic review.
Data sources: We searched PubMed and EMBASE databases for studies reported from January 2015 to December 2021.
Eligibility criteria: We included RCTs reported in English that used AI as the intervention. Protocols, conference abstracts, studies on robotics and studies related to medical education were excluded.
Data extraction: The included studies were graded using the CONSORT-AI checklist, comprising 43 items, by two independent graders. The results were tabulated and descriptive statistics were reported.
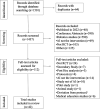
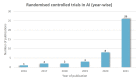
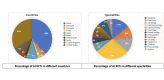
Results: We screened 1501 potential abstracts, of which 112 full-text articles were reviewed for eligibility. A total of 42 studies were included. The number of participants ranged from 22 to 2352. Only two items of the CONSORT-AI items were fully reported in all studies. Five items were not applicable in more than 85% of the studies. Nineteen per cent (8/42) of the studies did not report more than 50% (21/43) of the CONSORT-AI checklist items.
Conclusions: The quality of reporting of RCTs in AI is suboptimal. As reporting is variable in existing RCTs, caution should be exercised in interpreting the findings of some studies.
Keywords: clinical trials; health informatics; statistics & research methods.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
