Defining the lipidome of Arabidopsis leaf mitochondria: Specific lipid complement and biosynthesis capacity
- PMID: 36691154
- PMCID: PMC10069894
- DOI: 10.1093/plphys/kiad035
Defining the lipidome of Arabidopsis leaf mitochondria: Specific lipid complement and biosynthesis capacity
Abstract
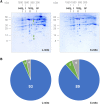
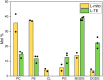
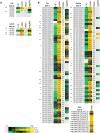
Mitochondria are often considered as the power stations of the cell, playing critical roles in various biological processes such as cellular respiration, photosynthesis, stress responses, and programmed cell death. To maintain the structural and functional integrities of mitochondria, it is crucial to achieve a defined membrane lipid composition between different lipid classes wherein specific proportions of individual lipid species are present. Although mitochondria are capable of self-synthesizing a few lipid classes, many phospholipids are synthesized in the endoplasmic reticulum and transferred to mitochondria via membrane contact sites, as mitochondria are excluded from the vesicular transportation pathway. However, knowledge on the capability of lipid biosynthesis in mitochondria and the precise mechanism of maintaining the homeostasis of mitochondrial lipids is still scarce. Here we describe the lipidome of mitochondria isolated from Arabidopsis (Arabidopsis thaliana) leaves, including the molecular species of glycerolipids, sphingolipids, and sterols, to depict the lipid landscape of mitochondrial membranes. In addition, we define proteins involved in lipid metabolism by proteomic analysis and compare our data with mitochondria from cell cultures since they still serve as model systems. Proteins putatively localized to the membrane contact sites are proposed based on the proteomic results and online databases. Collectively, our results suggest that leaf mitochondria are capable-with the assistance of membrane contact site-localized proteins-of generating several lipid classes including phosphatidylethanolamines, cardiolipins, diacylgalactosylglycerols, and free sterols. We anticipate our work to be a foundation to further investigate the functional roles of lipids and their involvement in biochemical reactions in plant mitochondria.
© The Author(s) 2023. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures






Similar articles
-
A combined lipidomic and proteomic profiling of Arabidopsis thaliana plasma membrane.Plant J. 2024 Aug;119(3):1570-1595. doi: 10.1111/tpj.16810. Epub 2024 May 18. Plant J. 2024. PMID: 38761101
-
Isolation of Mitochondria for Lipid Analysis.Methods Mol Biol. 2021;2295:337-349. doi: 10.1007/978-1-0716-1362-7_18. Methods Mol Biol. 2021. PMID: 34047985
-
Proteomic and lipidomic analyses of the Arabidopsis atg5 autophagy mutant reveal major changes in endoplasmic reticulum and peroxisome metabolisms and in lipid composition.New Phytol. 2019 Aug;223(3):1461-1477. doi: 10.1111/nph.15913. Epub 2019 Jun 22. New Phytol. 2019. PMID: 31077612
-
Intramitochondrial phospholipid trafficking.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Jan;1862(1):81-89. doi: 10.1016/j.bbalip.2016.08.006. Epub 2016 Aug 16. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 27542541 Review.
-
Mitochondrial lipid trafficking.Trends Cell Biol. 2014 Jan;24(1):44-52. doi: 10.1016/j.tcb.2013.07.011. Epub 2013 Sep 1. Trends Cell Biol. 2014. PMID: 24001776 Review.
Cited by
-
The de novo assembly and characterization of the complete mitochondrial genome of bottle gourd (Lagenaria siceraria) reveals the presence of homologous conformations produced by repeat-mediated recombination.Front Plant Sci. 2024 Aug 12;15:1416913. doi: 10.3389/fpls.2024.1416913. eCollection 2024. Front Plant Sci. 2024. PMID: 39188545 Free PMC article.
-
Exploring the plant lipidome: techniques, challenges, and prospects.Adv Biotechnol (Singap). 2024 Mar 11;2(1):11. doi: 10.1007/s44307-024-00017-9. Adv Biotechnol (Singap). 2024. PMID: 39883225 Free PMC article. Review.
-
Kingdom-specific lipid unsaturation calibrates sequence evolution in membrane arm subunits of eukaryotic respiratory complexes.Nat Commun. 2025 Feb 27;16(1):2044. doi: 10.1038/s41467-025-57295-7. Nat Commun. 2025. PMID: 40016208 Free PMC article.
-
Mitochondrial dysfunction in oocytes: implications for fertility and ageing.J Ovarian Res. 2025 Aug 14;18(1):186. doi: 10.1186/s13048-025-01764-6. J Ovarian Res. 2025. PMID: 40814060 Free PMC article. Review.
-
Arabidopsis Leaf Chloroplasts Have a Specific Sphingolipidome.Plants (Basel). 2024 Jan 19;13(2):299. doi: 10.3390/plants13020299. Plants (Basel). 2024. PMID: 38276756 Free PMC article.
References
-
- Achleitner G, Gaigg B, Krasser A, Kainersdorfer E, Kohlwein SD, Perktold A, Zellnig G, Daum G (1999) Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contact. Eur J Biochem 264(2): 545–553 - PubMed
-
- Alcázar-Román AR, Wente SR (2008) Inositol polyphosphates: a new frontier for regulating gene expression. Chromosoma 117(1): 1–13 - PubMed
-
- Ali U, Li H, Wang X, Guo L (2018) Emerging roles of sphingolipid signaling in plant response to biotic and abiotic stresses. Mol Plant 11(11): 1328–1343 - PubMed
-
- Babiychuk E, Müller F, Eubel H, Braun H-P, Frentzen M, Kushnir S (2003) Arabidopsis phosphatidylglycerophosphate synthase 1 is essential for chloroplast differentiation, but is dispensable for mitochondrial function. Plant J 33(5): 899–909 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

