Intraoperative visualisation of pancreatic leakage (ViP): study protocol for an IDEAL Stage I Post Market Clinical Study
- PMID: 36691219
- PMCID: PMC9462113
- DOI: 10.1136/bmjopen-2022-065157
Intraoperative visualisation of pancreatic leakage (ViP): study protocol for an IDEAL Stage I Post Market Clinical Study
Abstract
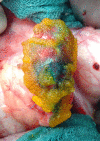
Introduction: Pancreatic resections are an important field of surgery worldwide to treat a variety of benign and malignant diseases. Postoperative pancreatic fistula (POPF) remains a frequent and critical complication after partial pancreatectomy and affects up to 50% of patients. POPF increases mortality, prolongs the postoperative hospital stay and is associated with a significant economic burden. Despite various scientific approaches and clinical strategies, it has not yet been possible to develop an effective preventive tool. The SmartPAN indicator is the first surgery-ready medical device for direct visualisation of pancreatic leakage already during the operation. Applied to the surface of pancreatic tissue, it detects sites of biochemical leak via colour reaction, thereby guiding effective closure and potentially mitigating POPF development.
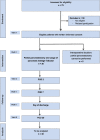
Methods and analysis: The ViP trial is a prospective single-arm, single-centre first in human study to collect data on usability and confirm safety of SmartPAN. A total of 35 patients with planned partial pancreatectomy will be included in the trial with a follow-up of 30 days after the index surgery. Usability endpoints such as adherence to protocol and evaluation by the operating surgeon as well as safety parameters including major intraoperative and postoperative complications, especially POPF development, will be analysed.
Ethics and dissemination: Following the IDEAL-D (Idea, Development, Exploration, Assessment, and Long term study of Device development and surgical innovation) framework of medical device development preclinical in vitro, porcine in vivo, and human ex vivo studies have proven feasibility, efficacy and safety of SmartPAN. After market approval, the ViP trial is the IDEAL Stage I trial to investigate SmartPAN in a clinical setting. The study has been approved by the local ethics committee as the device is used exclusively within its intended purpose. Results will be published in a peer-reviewed journal. The study will provide a basis for a future randomised controlled interventional trial to confirm clinical efficacy of SmartPAN.
Trial registration number: German Clinical Trial Register DRKS00027559, registered on 4 March 2022.
Keywords: IDEAL; SmartPAN; indicator; pancreas; partial pancreatectomy; postoperative pancreatic fistula; resection; surgery.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: TMP reports financial support provided by Heidelberger Stiftung Chirurgie. BG reports a relationship with Magle Chemoswed that includes employment. TMP and TH have patent Body Fluid Leakage Detection Aqueous Composition (Pausch T, Hackert T, Johansson H, et al. European Patent Office, Germany. 2018) licensed to Magle Chemoswed Holding AB and University of Heidelberg.
Figures
Similar articles
-
Visualization of Intraoperative Pancreatic Leakage (ViP): The IDEAL Stage I First-in-human, Single-arm Clinical Pilot Trial of SmartPAN.Ann Surg Open. 2025 Mar 11;6(1):e529. doi: 10.1097/AS9.0000000000000529. eCollection 2025 Mar. Ann Surg Open. 2025. PMID: 40134475 Free PMC article.
-
Cavitron ultrasonic surgical aspirator (CUSA) compared with conventional pancreatic transection in distal pancreatectomy: study protocol for the randomised controlled CUSA-1 pilot trial.BMJ Open. 2024 Apr 18;14(4):e082024. doi: 10.1136/bmjopen-2023-082024. BMJ Open. 2024. PMID: 38637127 Free PMC article.
-
SmartPAN: A novel polysaccharide-microsphere-based surgical indicator of pancreatic leakage.J Biomater Appl. 2020 Jul;35(1):123-134. doi: 10.1177/0885328220913057. Epub 2020 Mar 17. J Biomater Appl. 2020. PMID: 32183581
-
Attempts to prevent postoperative pancreatic fistula after distal pancreatectomy.Surg Today. 2017 Apr;47(4):416-424. doi: 10.1007/s00595-016-1367-8. Epub 2016 Jun 20. Surg Today. 2017. PMID: 27324393 Review.
-
Management of postoperative pancreatic fistula after pancreaticoduodenectomy.J Visc Surg. 2023 Feb;160(1):39-51. doi: 10.1016/j.jviscsurg.2023.01.002. Epub 2023 Jan 24. J Visc Surg. 2023. PMID: 36702720 Review.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources