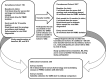
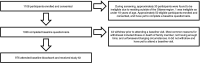
Cohort profile: S top the Spread Ottawa (SSO) - a community-based prospective cohort study on antibody responses, antibody neutralisation efficiency and cellular immunity to SARS-CoV-2 infection and vaccination
- PMID: 36691221
- PMCID: PMC9461086
- DOI: 10.1136/bmjopen-2022-062187
Cohort profile: S top the Spread Ottawa (SSO) - a community-based prospective cohort study on antibody responses, antibody neutralisation efficiency and cellular immunity to SARS-CoV-2 infection and vaccination
Abstract
Purpose: To investigate the robustness and longevity of SARS-CoV-2 immune responses conferred by natural infection and vaccination among priority populations such as immunocompromised individuals and people with post-acute sequelae of COVID-19 in a prospective cohort study (Stop the Spread Ottawa-SSO) in adults living in the Ottawa region. In this paper, we describe the study design, ongoing data collection and baseline characteristics of participants.
Participants: Since October 2020, participants who tested positive for COVID-19 (convalescents) or at high risk of exposure to the virus (under surveillance) have provided monthly blood and saliva samples over a 10-month period. As of 2 November 2021, 1026 adults had completed the baseline survey and 976 had attended baseline bloodwork. 300 participants will continue to provide bimonthly blood samples for 24 additional months (ie, total follow-up of 34 months).
Findings to date: The median age of the baseline sample was 44 (IQR 23, range: 18-79) and just over two-thirds (n=688; 67.1%) were female. 255 participants (24.9%) had a history of COVID-19 infection confirmed by PCR and/or serology. Over 600 participants (60.0%) work in high-risk occupations (eg, healthcare, teaching and transportation). 108 participants (10.5%) reported immunocompromising conditions or treatments at baseline (eg, cancer, HIV, other immune deficiency, and/or use of immunosuppressants).
Future plans: SSO continues to yield rich research potential, given the collection of pre-vaccine baseline data and samples from the majority of participants, recruitment of diverse subgroups of interest, and a high level of participant retention and compliance with monthly sampling. The 24-month study extension will maximise opportunities to track SARS-CoV-2 immunity and vaccine efficacy, detect and characterise emerging variants, and compare subgroup humoral and cellular response robustness and persistence.
Keywords: COVID-19; epidemiology; immunology; infectious diseases; public health; virology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Results of the Stop the Spread Ottawa (SSO) cohort study: a Canadian urban-based prospective evaluation of antibody responses and neutralisation efficiency to SARS-CoV-2 infection and vaccination.BMJ Open. 2023 Oct 31;13(10):e077714. doi: 10.1136/bmjopen-2023-077714. BMJ Open. 2023. PMID: 37907304 Free PMC article.
-
BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers.Lancet Respir Med. 2021 Sep;9(9):999-1009. doi: 10.1016/S2213-2600(21)00220-4. Epub 2021 Jul 2. Lancet Respir Med. 2021. PMID: 34224675 Free PMC article.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Cohort profile: evaluation of immune response and household transmission of SARS-CoV-2 in Costa Rica: the RESPIRA study.BMJ Open. 2023 Dec 9;13(12):e071284. doi: 10.1136/bmjopen-2022-071284. BMJ Open. 2023. PMID: 38070892 Free PMC article.
-
Cohort Profile:The Danish National Cohort Study of Effectiveness and Safety of SARS-CoV-2 vaccines (ENFORCE).BMJ Open. 2022 Dec 30;12(12):e069065. doi: 10.1136/bmjopen-2022-069065. BMJ Open. 2022. PMID: 36585137 Free PMC article.
Cited by
-
Reduced seasonal coronavirus incidence in high-risk population groups during the COVID-19 pandemic.Immun Inflamm Dis. 2024 Jul;12(7):e1342. doi: 10.1002/iid3.1342. Immun Inflamm Dis. 2024. PMID: 39023424 Free PMC article.
-
Multivariate analyses and machine learning link sex and age with antibody responses to SARS-CoV-2 and vaccination.iScience. 2024 Jul 10;27(8):110484. doi: 10.1016/j.isci.2024.110484. eCollection 2024 Aug 16. iScience. 2024. PMID: 39156648 Free PMC article.
-
Autoantibodies targeting angiotensin-converting enzyme 2 are prevalent and not induced by SARS-CoV-2 infection.FASEB J. 2025 Feb 28;39(4):e70390. doi: 10.1096/fj.202402694R. FASEB J. 2025. PMID: 39950298 Free PMC article.
-
SARS-CoV-2 Vaccine-Induced T-Cell Response after Three Doses in People Living with HIV on Antiretroviral Therapy Compared to Seronegative Controls (CTN 328 COVAXHIV Study).Viruses. 2023 Feb 19;15(2):575. doi: 10.3390/v15020575. Viruses. 2023. PMID: 36851789 Free PMC article.
-
Antibody neutralization capacity after coronavirus disease 2019 vaccination in people with HIV in Canada.AIDS. 2023 Oct 1;37(12):F25-F35. doi: 10.1097/QAD.0000000000003680. Epub 2023 Aug 2. AIDS. 2023. PMID: 37534695 Free PMC article.
References
-
- WHO . Coronavirus (COVID-19) Dashboard, 2022. Available: https://covid19.who.int/ [Accessed January 3, 2022].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous