Application of organoid culture from HPV18-positive small cell carcinoma of the uterine cervix for precision medicine
- PMID: 36691316
- PMCID: PMC10134306
- DOI: 10.1002/cam4.5588
Application of organoid culture from HPV18-positive small cell carcinoma of the uterine cervix for precision medicine
Abstract
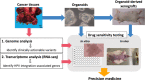
Background: Small cell carcinoma of the uterine cervix (SCCC) is a rare and highly malignant human papillomavirus (HPV)-associated cancer in which human genes related to the integration site can serve as a target for precision medicine. The aim of our study was to establish a workflow for precision medicine of HPV-associated cancer using patient-derived organoid.
Methods: Organoid was established from the biopsy of a patient diagnosed with HPV18-positive SCCC. Therapeutic targets were identified by whole exome sequencing (WES) and RNA-seq analysis. Drug sensitivity testing was performed using organoids and organoid-derived mouse xenograft model.
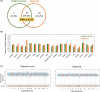
Results: WES revealed that both the original tumor and organoid had 19 somatic variants in common, including the KRAS p.G12D pathogenic variant. Meanwhile, RNA-seq revealed that HPV18 was integrated into chromosome 8 at 8q24.21 with increased expression of the proto-oncogene MYC. Drug sensitivity testing revealed that a KRAS pathway inhibitor exerted strong anti-cancer effects on the SCCC organoid compared to a MYC inhibitor, which were also confirmed in the xenograft model.
Conclusion: In this study, we confirmed two strategies for identifying therapeutic targets of HPV-derived SCCC, WES for identifying pathogenic variants and RNA sequencing for identifying HPV integration sites. Organoid culture is an effective tool for unveiling the oncogenic process of rare tumors and can be a breakthrough for the development of precision medicine for patients with HPV-positive SCCC.
Keywords: cervical cancer; human papillomavirus; integration site; organoid; precision medicine.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures




Similar articles
-
Identification of target cells of human papillomavirus 18 using squamocolumnar junction organoids.Cancer Sci. 2024 Jan;115(1):125-138. doi: 10.1111/cas.15988. Epub 2023 Nov 23. Cancer Sci. 2024. PMID: 37996972 Free PMC article.
-
Human papillomavirus integration perspective in small cell cervical carcinoma.Nat Commun. 2022 Oct 10;13(1):5968. doi: 10.1038/s41467-022-33359-w. Nat Commun. 2022. PMID: 36216793 Free PMC article.
-
Genomic characterization of small cell carcinomas of the uterine cervix.Mol Oncol. 2022 Feb;16(4):833-845. doi: 10.1002/1878-0261.12962. Epub 2021 May 13. Mol Oncol. 2022. PMID: 33830625 Free PMC article.
-
Small Cell and Other Rare Histologic Types of Cervical Cancer.Curr Oncol Rep. 2022 Nov;24(11):1531-1539. doi: 10.1007/s11912-022-01316-x. Epub 2022 Aug 10. Curr Oncol Rep. 2022. PMID: 35947285 Review.
-
Global Variation of Human Papillomavirus Genotypes and Selected Genes Involved in Cervical Malignancies.Ann Glob Health. 2015 Sep-Oct;81(5):675-83. doi: 10.1016/j.aogh.2015.08.026. Ann Glob Health. 2015. PMID: 27036725 Review.
Cited by
-
Endoplasmic reticulum stress in breast cancer: a predictive model for prognosis and therapy selection.Front Immunol. 2024 Feb 19;15:1332942. doi: 10.3389/fimmu.2024.1332942. eCollection 2024. Front Immunol. 2024. PMID: 38440732 Free PMC article.
-
Advancements in the Understanding of Small-Cell Neuroendocrine Cervical Cancer: Where We Stand and What Lies Ahead.J Pers Med. 2024 Apr 27;14(5):462. doi: 10.3390/jpm14050462. J Pers Med. 2024. PMID: 38793044 Free PMC article. Review.
-
Organoid modeling meets cancers of female reproductive tract.Cell Death Discov. 2024 Sep 27;10(1):410. doi: 10.1038/s41420-024-02186-x. Cell Death Discov. 2024. PMID: 39333482 Free PMC article. Review.
-
Targeting Metastasis: Exploring the Impact of Microbial Infections on Cancer Progression Through Innovative Biological Models.Curr Microbiol. 2025 Jun 7;82(7):328. doi: 10.1007/s00284-025-04306-x. Curr Microbiol. 2025. PMID: 40481883 Review.
-
Organoid technology in cervical cancer research.Am J Cancer Res. 2025 May 15;15(5):1988-2003. doi: 10.62347/FNTD1712. eCollection 2025. Am J Cancer Res. 2025. PMID: 40520874 Free PMC article. Review.

