Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs
- PMID: 36691401
- PMCID: PMC9850348
- DOI: 10.1038/s41578-022-00529-7
Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs
Abstract
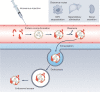
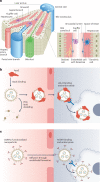
Genetic drugs based on nucleic acid biomolecules are a rapidly emerging class of medicines that directly reprogramme the central dogma of biology to prevent and treat disease. However, multiple biological barriers normally impede the intracellular delivery of nucleic acids, necessitating the use of a delivery system. Lipid and polymer nanoparticles represent leading approaches for the clinical translation of genetic drugs. These systems circumnavigate biological barriers and facilitate the intracellular delivery of nucleic acids in the correct cells of the target organ using passive, active and endogenous targeting mechanisms. In this Review, we highlight the constituent materials of these advanced nanoparticles, their nucleic acid cargoes and how they journey through the body. We discuss targeting principles for liver delivery, as it is the organ most successfully targeted by intravenously administered nanoparticles to date, followed by the expansion of these concepts to extrahepatic (non-liver) delivery. Ultimately, this Review connects emerging materials and biological insights playing key roles in targeting specific organs and cells in vivo.
Keywords: Biomedical engineering; Drug delivery.
© Springer Nature Limited 2023, Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Competing interestsD.J.S.: ReCode Therapeutics, co-founder, consultant and Scientific Advisory Board; Tome Biosciences, Scientific Advisory Board. D.J.S., S.A.D. and the Regents of the University of Texas System have filed patent applications related to delivery technologies.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
