Hypoxia-Induced Autophagy Is Involved in Radioresistance via HIF1A-Associated Beclin-1 in Glioblastoma Multiforme
- PMID: 36691538
- PMCID: PMC9860297
- DOI: 10.1016/j.heliyon.2023.e12820
Hypoxia-Induced Autophagy Is Involved in Radioresistance via HIF1A-Associated Beclin-1 in Glioblastoma Multiforme
Abstract
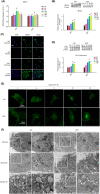
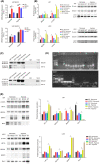
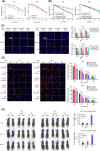
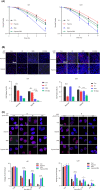
Radioresistance is the major factor of glioblastoma multiforme (GBM) treatment failure and relapse. Hypoxia and autophagy are linked to radioresistance and poor prognosis in solid tumors, but mechanisms remain unknown. Thus, we hypothesize that hypoxia may activate autophagy through two critical factors, HIF1A and Beclin-1, resulting in radioresistance of GBM in vitro and in vivo. In this study, we first demonstrated that HIF1A was overexpressed in GBM tissues and predicted a poor prognosis via bioinformatics. Secondly, we determined that hypoxia induced high expression of HIF1A and upregulated levels of Beclin-1 and autophagy, while HIF1A knockdown by shRNA reduced the expression of Beclin-1. Then we revealed the crosstalk and mechanisms of HIF1A-associated-Beclin-1 in three aspects: (a) transcriptional regulation, (b) protein interaction, and (c) HIF1A/BNIP3/Beclin-1 signaling pathway. Furthermore, we confirmed that silencing HIF1A enhanced the radiosensitivity of GBM in vitro and in vivo. Additionally, Beclin-1 suppression by 3-MA could reverse radioresistance induced by HIF1A under hypoxia. In conclusion, we demonstrated that hypoxia triggered autophagy via HIF1A-associated Beclin-1, resulting in radioresistance in GBM. HIF1A knockdown improved GBM radiosensitivity, and silencing Beclin-1 could reverse HIF1A-induced radioresistance under hypoxic conditions. These findings may help us comprehend the molecular underpinnings of hypoxia-induced autophagy and provide a novel perspective and prospective treatment for GBM radiosensitization.
Keywords: Autophagy; Beclin-1; Hypoxia; Hypoxia-inducible factor 1 alpha (HIF1A); Radiotherapy.
© 2023 The Authors.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

