Design and 3D printing of an electrochemical sensor for Listeria monocytogenes detection based on loop mediated isothermal amplification
- PMID: 36691544
- PMCID: PMC9860429
- DOI: 10.1016/j.heliyon.2022.e12637
Design and 3D printing of an electrochemical sensor for Listeria monocytogenes detection based on loop mediated isothermal amplification
Abstract
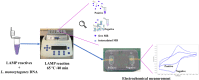
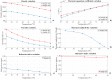
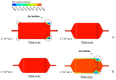
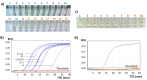
The aim of this work is the design and 3D printing of a new electrochemical sensor for the detection of Listeria monocytogenes based on loop mediated isothermal amplification (LAMP). The food related diseases involve a serious health issue all over the world. Listeria monocytogenes is one of the major problems of contaminated food, this pathogen causes a disease called listeriosis with a high rate of hospitalization and mortality. Having a fast, sensitive and specific detection method for food quality control is a must in the food industry to avoid the presence of this pathogen in the food chain (raw materials, facilities and products). A point-of-care biosensor based in LAMP and electrochemical detection is one of the best options to detect the bacteria on site and in a very short period of time. With the numerical analysis of different geometries and flow rates during sample injection in order to avoid bubbles, an optimized design of the microfluidic biosensor chamber was selected for 3D-printing and experimental analysis. For the electrochemical detection, a novel custom gold concentric-3-electrode consisting in a working electrode, reference electrode and a counter electrode was designed and placed in the bottom of the chamber. The LAMP reaction was optimized specifically for a primers set with a limit of detection of 1.25 pg of genomic DNA per reaction and 100% specific for detecting all 12 Listeria monocytogenes serotypes and no other Listeria species or food-related bacteria. The methylene blue redox-active molecule was tested as the electrochemical transducer and shown to be compatible with the LAMP reaction and very clearly distinguished negative from positive food samples when the reaction is measured at the end-point inside the biosensor.
Keywords: 3D printing; Biosensor; Electrochemical detection; Listeria monocytogenes; Loop-mediated isothermal AMPlification (LAMP); Numerical analysis.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Development of a loop-mediated isothermal amplification assay targeting lmo0753 gene for detection of Listeria monocytogenes in wastewater.Lett Appl Microbiol. 2019 Oct;69(4):264-270. doi: 10.1111/lam.13200. Epub 2019 Aug 9. Lett Appl Microbiol. 2019. PMID: 31323126
-
Development of loop-mediated isothermal amplification-lateral flow dipstick as a rapid screening test for detecting Listeria monocytogenes in frozen food products using a specific region on the ferrous iron transport protein B gene.Vet World. 2022 Mar;15(3):590-601. doi: 10.14202/vetworld.2022.590-601. Epub 2022 Mar 12. Vet World. 2022. PMID: 35497940 Free PMC article.
-
Mpl-Gene-Based Loop-Mediated Isothermal Amplification Assay for Specific and Rapid Detection of Listeria monocytogenes in Various Food Samples.Foodborne Pathog Dis. 2022 Jul;19(7):463-472. doi: 10.1089/fpd.2021.0080. Epub 2022 Jan 28. Foodborne Pathog Dis. 2022. PMID: 35099299
-
A Label-Based Polymer Nanoparticles Biosensor Combined with Loop-Mediated Isothermal Amplification for Rapid, Sensitive, and Highly Specific Identification of Brucella abortus.Front Bioeng Biotechnol. 2021 Nov 18;9:758564. doi: 10.3389/fbioe.2021.758564. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34869267 Free PMC article. Review.
-
Biosensor for the detection of Listeria monocytogenes: emerging trends.Crit Rev Microbiol. 2018 Sep;44(5):590-608. doi: 10.1080/1040841X.2018.1473331. Epub 2018 May 23. Crit Rev Microbiol. 2018. PMID: 29790396 Review.
Cited by
-
Development of an Electrochemical Sensor for SARS-CoV-2 Detection Based on Loop-Mediated Isothermal Amplification.Biosensors (Basel). 2023 Oct 11;13(10):924. doi: 10.3390/bios13100924. Biosensors (Basel). 2023. PMID: 37887117 Free PMC article.
-
Extraction-Free Colorimetric RT-LAMP Detection of SARS-CoV-2 in Saliva.Diagnostics (Basel). 2023 Jul 11;13(14):2344. doi: 10.3390/diagnostics13142344. Diagnostics (Basel). 2023. PMID: 37510088 Free PMC article.
-
Advancements and applications of loop-mediated isothermal amplification technology: a comprehensive overview.Front Microbiol. 2024 Jul 17;15:1406632. doi: 10.3389/fmicb.2024.1406632. eCollection 2024. Front Microbiol. 2024. PMID: 39091309 Free PMC article. Review.
-
Fabrication of a Microfluidic Test Device with a 3D Printer and Its Combination with the Loop Mediated Isothermal Amplification Method to Detect Streptococcus pyogenes.Micromachines (Basel). 2024 Mar 7;15(3):365. doi: 10.3390/mi15030365. Micromachines (Basel). 2024. PMID: 38542612 Free PMC article.
-
Polymeric and Paper-Based Lab-on-a-Chip Devices in Food Safety: A Review.Micromachines (Basel). 2023 Apr 30;14(5):986. doi: 10.3390/mi14050986. Micromachines (Basel). 2023. PMID: 37241610 Free PMC article. Review.
References
-
- Wu M.Y.-C., Hsu M.-Y., Chen S.-J., Hwang D.-K., Yen T.-H., Cheng C.-M. Point-of-Care detection devices for food safety monitoring: proactive disease prevention. Trends Biotechnol. 2017;35:288–300. - PubMed
-
- Surveillance for foodborne disease outbreaks United States, 2017: annual report. 2017. http://www.cdc.gov/foodsafety/fdoss/ (accessed February 24, 2022)
-
- Soni D.K., Ahmad R., Dubey S.K. Biosensor for the detection of Listeria monocytogenes: emerging trends. Crit. Rev. Microbiol. 2018;44:590–608. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

