Theoretical study on the radical scavenging activity of gallic acid
- PMID: 36691549
- PMCID: PMC9860461
- DOI: 10.1016/j.heliyon.2023.e12806
Theoretical study on the radical scavenging activity of gallic acid
Abstract
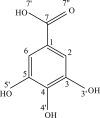
The global descriptors of the chemical activity: ionization potential IP, electron affinity EA, chemical potential μ, absolute electronegativity χ, molecular hardness η and softness S, electrophilicity index ω, electro-donating ω-, electro-accepting ω+ powers as well as Ra and Rd indexes for gallic acid (GA) in the gas phase and water medium have been determined. To this aim, the HOMO and LUMO energies were calculated using the DFT method at the B3LYP, M06-2X, LC-ωPBE, BHandLYP, ωB97XD/cc-pVQZ theory levels using C-PCM, IEF-PCM and SMD solvation models, enabling more accurate descriptor calculations than those carried out so far. Quantum-chemical computations were also applied to investigate the GA structure and thermodynamic parameters characterizing its radical scavenging properties. To this aim, the full optimization of the neutral GA and its radical, cationic and anionic forms in vacuum and water medium has been performed, and then the bond dissociation enthalpy BDE, adiabatic ionization potential AIP, proton dissociation enthalpy PDE, proton affinity PA, electron transfer enthalpy ETE, gas phase acidity Hacidity and free Gibbs acidity Gacidity in water have been determined. The calculations revealed that GA in vacuum scavenges free radicals via hydrogen atom transfer (HAT), whereas in water (polar) medium by sequential proton loss electron transfer (SPLET). Analysis of the global activity descriptors of GA indicates that only B3LYP method combined with different solvation models satisfactory reproduces LUMO-HOMO energies and provides the smallest value of the total electron energy of GA. Among the parameters of chemical activity, the indexes Ra and Rd are the most independent of the computational method and the solvation model used. They can be recommended as a reliable source of information on the antioxidant activity of chemical compounds.
Keywords: AIP; Antioxidants; BDE; DFT method; ETE; Gallic acid; Global descriptors; PA; PDE; Solvation models; TMC descriptors.
© 2023 The Author. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Density Functional Theory Studies on the Chemical Reactivity of Allyl Mercaptan and Its Derivatives.Molecules. 2024 Jan 31;29(3):668. doi: 10.3390/molecules29030668. Molecules. 2024. PMID: 38338412 Free PMC article.
-
Free radical scavenging mechanism of 1,3,4-oxadiazole derivatives: thermodynamics of O-H and N-H bond cleavage.Heliyon. 2020 Mar 31;6(3):e03683. doi: 10.1016/j.heliyon.2020.e03683. eCollection 2020 Mar. Heliyon. 2020. PMID: 32258501 Free PMC article.
-
Two Theorems and Important Insight on How the Preferred Mechanism of Free Radical Scavenging Cannot Be Settled. Comment on Pandithavidana, D.R.; Jayawardana, S.B. Comparative Study of Antioxidant Potential of Selected Dietary Vitamins; Computational Insights. Molecules 2019, 24, 1646.Molecules. 2022 Nov 21;27(22):8092. doi: 10.3390/molecules27228092. Molecules. 2022. PMID: 36432191 Free PMC article.
-
PM6 study of free radical scavenging mechanisms of flavonoids: why does O-H bond dissociation enthalpy effectively represent free radical scavenging activity?J Mol Model. 2013 Jun;19(6):2593-603. doi: 10.1007/s00894-013-1800-5. Epub 2013 Mar 12. J Mol Model. 2013. PMID: 23479282
-
Recent advances in the antioxidant activity and mechanisms of chalcone derivatives: a computational review.Free Radic Res. 2022 May-Jun;56(5-6):378-397. doi: 10.1080/10715762.2022.2120396. Epub 2022 Sep 8. Free Radic Res. 2022. PMID: 36063087 Review.
Cited by
-
Density Functional Theory Studies on the Chemical Reactivity of Allyl Mercaptan and Its Derivatives.Molecules. 2024 Jan 31;29(3):668. doi: 10.3390/molecules29030668. Molecules. 2024. PMID: 38338412 Free PMC article.
-
α-Tocopherol and Trolox as Effective Natural Additives for Polyurethane Foams: A DFT and Experimental Study.Molecules. 2024 Dec 21;29(24):6037. doi: 10.3390/molecules29246037. Molecules. 2024. PMID: 39770125 Free PMC article.
-
Storage Time in Bottle: Influence on Physicochemical and Phytochemical Characteristics of Wine Spirits Aged Using Traditional and Alternative Technologies.Molecules. 2025 Apr 30;30(9):2018. doi: 10.3390/molecules30092018. Molecules. 2025. PMID: 40363823 Free PMC article.
-
Comparative study of physico-chemical properties of some molecules from Khaya Grandifoliola plant.Sci Rep. 2025 Mar 26;15(1):10430. doi: 10.1038/s41598-025-88302-y. Sci Rep. 2025. PMID: 40140436 Free PMC article.
-
Computation of the pKa Values of Gallic Acid and Its Anionic Forms in Aqueous Solution: A Self-Similar Transformation Approach for Accurate Proton Hydration Free Energy Estimation.Molecules. 2025 Feb 6;30(3):742. doi: 10.3390/molecules30030742. Molecules. 2025. PMID: 39942844 Free PMC article.
References
-
- Huang M.T., Chang R.L., Wood A.W., Newmark H.L., Sayer J.M., Yagi H., Jerina D.M., Conney A.H. Inhibition of mutagenecity of bay-region diol-epoxides of polycyclic aromatic hydrocarbons by tannic acid, hydroxylated anthraquinones and hydroxylated cinnamic acid derivatives. Carcinogenesis. 1995;6:237–242. doi: 10.1093/carcin/6.2.237. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

