Activation of NRF2 blocks HIV replication and apoptosis in macrophages
- PMID: 36691556
- PMCID: PMC9860420
- DOI: 10.1016/j.heliyon.2022.e12575
Activation of NRF2 blocks HIV replication and apoptosis in macrophages
Abstract
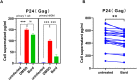
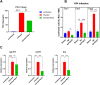
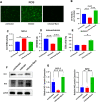
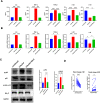
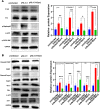
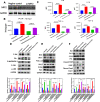
Abnormal oxidative stress caused by human immunodeficiency virus (HIV) infection affects viral replication and causes non-acquired immune deficiency syndrome-related complications in infected individuals. The transcription factor NFE2-related factor 2 (NRF2), a key regulator of oxidative stress, responds to abnormal oxidative stress by regulating the expression of NRF2-dependent cytoprotective genes. The present study aimed to determine whether inhibition of oxidative stress could control HIV replication and improve cell survival. In this study, the NRF2 activator, methyl bardoxolone, was used to treat cells for HIV infection. The effects on HIV replication and apoptosis pathways were confirmed by NRF2 activation or knockdown. The results showed that NRF2 activation could block HIV replication in macrophages before the integration phase and inhibited the expression of apoptotic pathways in virus-exposed macrophages. The study presents an unconventional anti-viral strategy of activation antioxidant response for HIV infection blocking.
Keywords: Apoptosis; HIV; Inflammation; Macrophage; Oxidative stress.
© 2022 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
HIV-1 decreases Nrf2/ARE activity and phagocytic function in alveolar macrophages.J Leukoc Biol. 2017 Aug;102(2):517-525. doi: 10.1189/jlb.4A0616-282RR. Epub 2017 May 26. J Leukoc Biol. 2017. PMID: 28550120 Free PMC article.
-
Induction of Heme Oxygenase-1 Deficiency and Associated Glutamate-Mediated Neurotoxicity Is a Highly Conserved HIV Phenotype of Chronic Macrophage Infection That Is Resistant to Antiretroviral Therapy.J Virol. 2015 Oct;89(20):10656-67. doi: 10.1128/JVI.01495-15. Epub 2015 Aug 12. J Virol. 2015. PMID: 26269184 Free PMC article.
-
Dengue Virus Targets Nrf2 for NS2B3-Mediated Degradation Leading to Enhanced Oxidative Stress and Viral Replication.J Virol. 2020 Nov 23;94(24):e01551-20. doi: 10.1128/JVI.01551-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32999020 Free PMC article.
-
Macrophages and HIV infection: therapeutical approaches toward this strategic virus reservoir.Antiviral Res. 2002 Aug;55(2):209-25. doi: 10.1016/s0166-3542(02)00052-9. Antiviral Res. 2002. PMID: 12103427 Review.
-
The role of oxidative stress in disease progression in individuals infected by the human immunodeficiency virus.J Leukoc Biol. 1992 Jul;52(1):111-4. doi: 10.1002/jlb.52.1.111. J Leukoc Biol. 1992. PMID: 1640166 Review.
Cited by
-
The Role of the NRF2 Pathway in the Pathogenesis of Viral Respiratory Infections.Pathogens. 2023 Dec 31;13(1):39. doi: 10.3390/pathogens13010039. Pathogens. 2023. PMID: 38251346 Free PMC article. Review.
-
The NLRP3 inflammasome and gut dysbiosis as a putative link between HIV-1 infection and ischemic stroke.Trends Neurosci. 2023 Aug;46(8):682-693. doi: 10.1016/j.tins.2023.05.009. Epub 2023 Jun 15. Trends Neurosci. 2023. PMID: 37330380 Free PMC article. Review.
-
Elevated Methylglyoxal: An Elusive Risk Factor Responsible for Early-Onset Cardiovascular Diseases in People Living with HIV-1 Infection.Viruses. 2025 Apr 8;17(4):547. doi: 10.3390/v17040547. Viruses. 2025. PMID: 40284990 Free PMC article. Review.
-
Shrimp Virus Regulates ROS Dynamics via the Nrf2 Pathway to Facilitate Viral Replication.Adv Sci (Weinh). 2025 May;12(18):e2407695. doi: 10.1002/advs.202407695. Epub 2025 Mar 16. Adv Sci (Weinh). 2025. PMID: 40091388 Free PMC article.
-
Quantitative Proteomics Reveal That CB2R Agonist JWH-133 Downregulates NF-κB Activation, Oxidative Stress, and Lysosomal Exocytosis from HIV-Infected Macrophages.Int J Mol Sci. 2024 Mar 13;25(6):3246. doi: 10.3390/ijms25063246. Int J Mol Sci. 2024. PMID: 38542221 Free PMC article.
References
-
- Phillips D.M., Tan X., Perotti M.E., Zacharopoulos V.R. Mechanism of monocyte-macrophage-mediated transmission of HIV. AIDS Res. Hum. Retrovir. 1998;14(Suppl 1):S67–70. - PubMed
LinkOut - more resources
Full Text Sources

