A pleiotropic chemoreceptor facilitates the production and perception of mating pheromones
- PMID: 36691619
- PMCID: PMC9860498
- DOI: 10.1016/j.isci.2022.105882
A pleiotropic chemoreceptor facilitates the production and perception of mating pheromones
Abstract
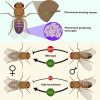
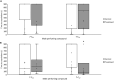
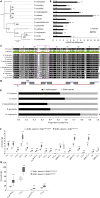
Optimal mating decisions depend on the robust coupling of signal production and perception because independent changes in either could carry a fitness cost. However, since the perception and production of mating signals are often mediated by different tissues and cell types, the mechanisms that drive and maintain their coupling remain unknown for most animal species. Here, we show that in Drosophila, behavioral responses to, and the production of, a putative inhibitory mating pheromone are co-regulated by Gr8a, a member of the Gustatory receptor gene family. Specifically, through behavioral and pheromonal data, we found that Gr8a independently regulates the behavioral responses of males and females to a putative inhibitory pheromone, as well as its production in the fat body and oenocytes of males. Overall, these findings provide a relatively simple molecular explanation for how pleiotropic receptors maintain robust mating signaling systems at the population and species levels.
Keywords: Biochemistry; Biological sciences; Ecological biochemistry.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Contribution of oenocytes and pheromones to courtship behaviour in Drosophila.BMC Biochem. 2009 Aug 11;10:21. doi: 10.1186/1471-2091-10-21. BMC Biochem. 2009. PMID: 19671131 Free PMC article.
-
Pheromonal and behavioral cues trigger male-to-female aggression in Drosophila.PLoS Biol. 2010 Nov 23;8(11):e1000541. doi: 10.1371/journal.pbio.1000541. PLoS Biol. 2010. PMID: 21124886 Free PMC article.
-
A Drosophila protein family implicated in pheromone perception is related to Tay-Sachs GM2-activator protein.J Biol Chem. 2009 Jan 2;284(1):585-594. doi: 10.1074/jbc.M806474200. Epub 2008 Oct 24. J Biol Chem. 2009. PMID: 18952610 Free PMC article.
-
Sexual attraction: on the role of fungal pheromone/receptor systems (A review).Acta Microbiol Immunol Hung. 2008 Jun;55(2):125-43. doi: 10.1556/AMicr.55.2008.2.5. Acta Microbiol Immunol Hung. 2008. PMID: 18595318 Review.
-
Pheromonal control: reconciling physiological mechanism with signalling theory.Biol Rev Camb Philos Soc. 2015 May;90(2):542-59. doi: 10.1111/brv.12123. Epub 2014 Jun 13. Biol Rev Camb Philos Soc. 2015. PMID: 24925630 Review.
Cited by
-
The dual coding of a single sex pheromone receptor in Asian honeybee Apis cerana.Commun Biol. 2024 Apr 25;7(1):502. doi: 10.1038/s42003-024-06206-5. Commun Biol. 2024. PMID: 38664580 Free PMC article.
-
Function and evolution of Ir52 receptors in mate detection in Drosophila.Curr Biol. 2024 Dec 2;34(23):5395-5408.e6. doi: 10.1016/j.cub.2024.10.001. Epub 2024 Oct 28. Curr Biol. 2024. PMID: 39471807
-
Integrating lipid metabolism, pheromone production and perception by Fruitless and Hepatocyte Nuclear Factor 4.Sci Adv. 2023 Jun 30;9(26):eadf6254. doi: 10.1126/sciadv.adf6254. Epub 2023 Jun 30. Sci Adv. 2023. PMID: 37390217 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

