Graded spikes differentially signal neurotransmitter input in cerebrospinal fluid contacting neurons of the mouse spinal cord
- PMID: 36691620
- PMCID: PMC9860393
- DOI: 10.1016/j.isci.2022.105914
Graded spikes differentially signal neurotransmitter input in cerebrospinal fluid contacting neurons of the mouse spinal cord
Abstract
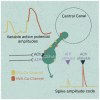
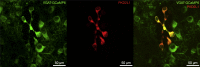
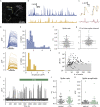
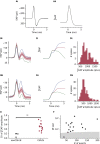
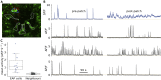
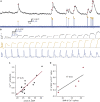
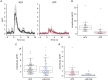
The action potential and its all-or-none nature is fundamental to neural communication. Canonically, the action potential is initiated once voltage-activated Na+ channels are activated, and their rapid kinetics of activation and inactivation give rise to the action potential's all-or-none nature. Here we demonstrate that cerebrospinal fluid contacting neurons (CSFcNs) surrounding the central canal of the mouse spinal cord employ a different strategy. Rather than using voltage-activated Na+ channels to generate binary spikes, CSFcNs use two different types of voltage-activated Ca2+ channel, enabling spikes of different amplitude. T-type Ca2+ channels generate small amplitude spikes, whereas larger amplitude spikes require high voltage-activated Cd2+-sensitive Ca2+ channels. We demonstrate that these different amplitude spikes can signal input from different transmitter systems; purinergic inputs evoke smaller T-type dependent spikes whereas cholinergic inputs evoke larger spikes that do not rely on T-type channels. Different synaptic inputs to CSFcNs can therefore be signaled by the spike amplitude.
Keywords: Cellular neuroscience; Molecular neuroscience; Systems neuroscience.
© 2023 The Authors.
Conflict of interest statement
E.J. is currently employed by ApconiX. The authors declare no competing interests.
Figures

Similar articles
-
Current clamp and modeling studies of low-threshold calcium spikes in cells of the cat's lateral geniculate nucleus.J Neurophysiol. 1999 May;81(5):2360-73. doi: 10.1152/jn.1999.81.5.2360. J Neurophysiol. 1999. PMID: 10322072
-
Simulation of Ca2+ persistent inward currents in spinal motoneurones: mode of activation and integration of synaptic inputs.J Physiol. 2006 Jan 15;570(Pt 2):355-74. doi: 10.1113/jphysiol.2005.099119. Epub 2005 Nov 24. J Physiol. 2006. PMID: 16308349 Free PMC article.
-
GABAB receptors modulate Ca2+ but not G protein-gated inwardly rectifying K+ channels in cerebrospinal-fluid contacting neurones of mouse brainstem.J Physiol. 2019 Jan;597(2):631-651. doi: 10.1113/JP277172. Epub 2018 Dec 10. J Physiol. 2019. PMID: 30418666 Free PMC article.
-
Breaking the code: regulation of neuronal differentiation by spontaneous calcium transients.Dev Neurosci. 1997;19(1):33-41. doi: 10.1159/000111183. Dev Neurosci. 1997. PMID: 9078431 Review.
-
Intrinsic and integrative properties of substantia nigra pars reticulata neurons.Neuroscience. 2011 Dec 15;198:69-94. doi: 10.1016/j.neuroscience.2011.07.061. Epub 2011 Aug 2. Neuroscience. 2011. PMID: 21839148 Free PMC article. Review.
Cited by
-
Endogenous opioid signalling regulates spinal ependymal cell proliferation.Nature. 2024 Oct;634(8033):407-414. doi: 10.1038/s41586-024-07889-w. Epub 2024 Sep 18. Nature. 2024. PMID: 39294372
-
Cerebrospinal fluid-contacting neurons: multimodal cells with diverse roles in the CNS.Nat Rev Neurosci. 2023 Sep;24(9):540-556. doi: 10.1038/s41583-023-00723-8. Epub 2023 Aug 9. Nat Rev Neurosci. 2023. PMID: 37558908 Review.
-
Cerebrospinal fluid-contacting neurons are sensory neurons with uniform morphological and region-specific electrophysiological properties in the mouse spinal cord.Commun Biol. 2025 Aug 15;8(1):1233. doi: 10.1038/s42003-025-08559-x. Commun Biol. 2025. PMID: 40817183 Free PMC article.
-
A Comparison of PKD2L1-Expressing Cerebrospinal Fluid Contacting Neurons in Spinal Cords of Rodents, Carnivores, and Primates.Int J Mol Sci. 2023 Sep 1;24(17):13582. doi: 10.3390/ijms241713582. Int J Mol Sci. 2023. PMID: 37686387 Free PMC article.
References
-
- Djenoune L., Khabou H., Joubert F., Quan F.B., Nunes Figueiredo S., Bodineau L., Del Bene F., Burcklé C., Tostivint H., Wyart C. Investigation of spinal cerebrospinal fluid-contacting neurons expressing PKD2L1: evidence for a conserved system from fish to primates. Front. Neuroanat. 2014;8:26. doi: 10.3389/fnana.2014.00026. - DOI - PMC - PubMed
-
- Kolmer W. Das “sagittalorgan” der Wirbeltiere. Z. Anat. Entwickl. Gesch. 1921;60:652–717. doi: 10.1007/bf02593657. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

