A Heterocatalytic Metal-Organic Framework to Stimulate Dispersal and Macrophage Combat with Infectious Biofilms
- PMID: 36692081
- PMCID: PMC9933606
- DOI: 10.1021/acsnano.2c09008
A Heterocatalytic Metal-Organic Framework to Stimulate Dispersal and Macrophage Combat with Infectious Biofilms
Abstract
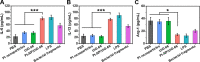
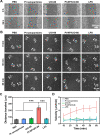
Eradication of infectious biofilms is becoming increasingly difficult due to the growing number of antibiotic-resistant strains. This necessitates development of nonantibiotic-based, antimicrobial approaches. To this end, we designed a heterocatalytic metal-organic framework composed of zirconium 1,4-dicarboxybenzene (UiO-66) with immobilized Pt nanoparticles (Pt-NP/UiO-66). Pt-NP/UiO-66 enhanced singlet-oxygen generation compared with Pt nanoparticles or UiO-66, particularly in an acidic environment. Singlet-oxygen generation degraded phosphodiester bonds present in eDNA gluing biofilms together and therewith dispersed biofilms. Remaining biofilms possessed a more open structure. Concurrently, Pt-NP/UiO-66 stimulated macrophages to adapt a more M1-like, "fighting" phenotype, moving faster toward their target bacteria and showing increased bacterial killing. As a combined effect of biofilm dispersal and macrophage polarization, a subcutaneous Staphylococcus aureus biofilm in mice was more readily eradicated by Pt-NP/UiO-66 than by Pt nanoparticles or UiO-66. Therewith, heterocatalytic Pt-NP/UiO-66 metal-organic frameworks constitute a nonantibiotic-based strategy to weaken protective matrices and disperse infectious biofilms, while strengthening macrophages in bacterial killing.
Keywords: Metal organic framework; antibacterial; extracellular DNA; immunomodulation; wound healing.
Conflict of interest statement
The authors declare the following competing financial interest(s): H.J.B. is also the director of a consulting company, SASA BV (GN Schutterlaan 4, 9797 PC Thesinge, The Netherlands). The authors declare no potential conflicts of interest with respect to authorship and/or publication of this Article. Opinions and assertions contained herein are those of the authors and are not construed as necessarily representing views of their respective employers.
Figures





Similar articles
-
Core-Shell ZnO2@Cerium-Based Metal-Organic Framework with Low Turnover, Dual-Catalytic Activity for Biosafe Biofilm Dispersal and Immune Modulation.ACS Appl Mater Interfaces. 2025 Jun 4;17(22):32111-32126. doi: 10.1021/acsami.5c08974. Epub 2025 May 21. ACS Appl Mater Interfaces. 2025. PMID: 40397809 Free PMC article.
-
Antimicrobial and antibiofilm activity of prepared thymol@UIO-66 and thymol/ZnONPs@UIO-66 nanoparticles against Methicillin-resistant Staphylococcus aureus: A synergistic approach.Colloids Surf B Biointerfaces. 2025 May;249:114529. doi: 10.1016/j.colsurfb.2025.114529. Epub 2025 Jan 20. Colloids Surf B Biointerfaces. 2025. PMID: 39879671
-
Photodynamic Inactivation of Bacteria and Biofilms with Benzoselenadiazole-Doped Metal-Organic Frameworks.Molecules. 2022 Dec 14;27(24):8908. doi: 10.3390/molecules27248908. Molecules. 2022. PMID: 36558041 Free PMC article.
-
Enzyme Mimicry for Combating Bacteria and Biofilms.Acc Chem Res. 2018 Mar 20;51(3):789-799. doi: 10.1021/acs.accounts.8b00011. Epub 2018 Feb 28. Acc Chem Res. 2018. PMID: 29489323 Review.
-
Antibacterial applications of metal-organic frameworks and their composites.Compr Rev Food Sci Food Saf. 2020 Jul;19(4):1397-1419. doi: 10.1111/1541-4337.12515. Epub 2020 Jan 7. Compr Rev Food Sci Food Saf. 2020. PMID: 33337086 Review.
Cited by
-
A normalized parameter for comparison of biofilm dispersants in vitro.Biofilm. 2024 Mar 3;7:100188. doi: 10.1016/j.bioflm.2024.100188. eCollection 2024 Jun. Biofilm. 2024. PMID: 38495770 Free PMC article.
-
Core-Shell ZnO2@Cerium-Based Metal-Organic Framework with Low Turnover, Dual-Catalytic Activity for Biosafe Biofilm Dispersal and Immune Modulation.ACS Appl Mater Interfaces. 2025 Jun 4;17(22):32111-32126. doi: 10.1021/acsami.5c08974. Epub 2025 May 21. ACS Appl Mater Interfaces. 2025. PMID: 40397809 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

