Hierarchical architecture of dopaminergic circuits enables second-order conditioning in Drosophila
- PMID: 36692262
- PMCID: PMC9937650
- DOI: 10.7554/eLife.79042
Hierarchical architecture of dopaminergic circuits enables second-order conditioning in Drosophila
Abstract
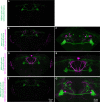
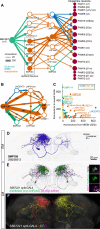
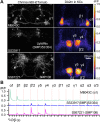
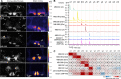
Dopaminergic neurons with distinct projection patterns and physiological properties compose memory subsystems in a brain. However, it is poorly understood whether or how they interact during complex learning. Here, we identify a feedforward circuit formed between dopamine subsystems and show that it is essential for second-order conditioning, an ethologically important form of higher-order associative learning. The Drosophila mushroom body comprises a series of dopaminergic compartments, each of which exhibits distinct memory dynamics. We find that a slow and stable memory compartment can serve as an effective 'teacher' by instructing other faster and transient memory compartments via a single key interneuron, which we identify by connectome analysis and neurotransmitter prediction. This excitatory interneuron acquires enhanced response to reward-predicting odor after first-order conditioning and, upon activation, evokes dopamine release in the 'student' compartments. These hierarchical connections between dopamine subsystems explain distinct properties of first- and second-order memory long known by behavioral psychologists.
Keywords: D. melanogaster; EM connectome; associative learning; dopamine; higher order conditioning; mushroom body; neural circuits; neuroscience.
© 2023, Yamada et al.
Conflict of interest statement
DY, DB, FL, KH, MS, JF, AL, TH, YA No competing interests declared
Figures














Similar articles
-
A dopamine-gated learning circuit underpins reproductive state-dependent odor preference in Drosophila females.Elife. 2022 Sep 21;11:e77643. doi: 10.7554/eLife.77643. Elife. 2022. PMID: 36129174 Free PMC article.
-
Distinct dopamine neurons mediate reward signals for short- and long-term memories.Proc Natl Acad Sci U S A. 2015 Jan 13;112(2):578-83. doi: 10.1073/pnas.1421930112. Epub 2014 Dec 29. Proc Natl Acad Sci U S A. 2015. PMID: 25548178 Free PMC article.
-
Nitric oxide acts as a cotransmitter in a subset of dopaminergic neurons to diversify memory dynamics.Elife. 2019 Nov 14;8:e49257. doi: 10.7554/eLife.49257. Elife. 2019. PMID: 31724947 Free PMC article.
-
Roles of feedback and feed-forward networks of dopamine subsystems: insights from Drosophila studies.Learn Mem. 2024 Jun 11;31(5):a053807. doi: 10.1101/lm.053807.123. Print 2024 May. Learn Mem. 2024. PMID: 38862171 Free PMC article. Review.
-
A non-canonical on-demand dopaminergic transmission underlying olfactory aversive learning.Neurosci Res. 2022 May;178:1-9. doi: 10.1016/j.neures.2021.12.008. Epub 2021 Dec 29. Neurosci Res. 2022. PMID: 34973292 Review.
Cited by
-
Sensory encoding and memory in the mushroom body: signals, noise, and variability.Learn Mem. 2024 Jun 11;31(5):a053825. doi: 10.1101/lm.053825.123. Print 2024 May. Learn Mem. 2024. PMID: 38862174 Free PMC article. Review.
-
Connectomics and the neural basis of behaviour.Curr Opin Insect Sci. 2022 Dec;54:100968. doi: 10.1016/j.cois.2022.100968. Epub 2022 Sep 13. Curr Opin Insect Sci. 2022. PMID: 36113710 Free PMC article. Review.
-
New genetic tools for mushroom body output neurons in Drosophila.Elife. 2024 Jan 25;12:RP90523. doi: 10.7554/eLife.90523. Elife. 2024. PMID: 38270577 Free PMC article.
-
A split-GAL4 driver line resource for Drosophila neuron types.Elife. 2025 Jan 24;13:RP98405. doi: 10.7554/eLife.98405. Elife. 2025. PMID: 39854223 Free PMC article.
-
Dopamine-Dependent Plasticity Is Heterogeneously Expressed by Presynaptic Calcium Activity across Individual Boutons of the Drosophila Mushroom Body.eNeuro. 2023 Oct 30;10(10):ENEURO.0275-23.2023. doi: 10.1523/ENEURO.0275-23.2023. Print 2023 Oct. eNeuro. 2023. PMID: 37848287 Free PMC article.
References
-
- Aso Y, Ray RP, Long X, Bushey D, Cichewicz K, Ngo TT, Sharp B, Christoforou C, Hu A, Lemire AL, Tillberg P, Hirsh J, Litwin-Kumar A, Rubin GM. Nitric oxide acts as a cotransmitter in a subset of dopaminergic neurons to diversify memory dynamics. eLife. 2019;8:e49257. doi: 10.7554/eLife.49257. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

