Novel Prion Strain as Cause of Chronic Wasting Disease in a Moose, Finland
- PMID: 36692340
- PMCID: PMC9881765
- DOI: 10.3201/eid2902.220882
Novel Prion Strain as Cause of Chronic Wasting Disease in a Moose, Finland
Abstract
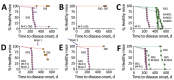
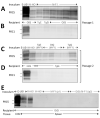
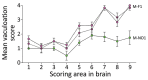
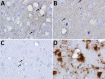
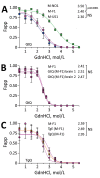
Our previous studies using gene-targeted mouse models of chronic wasting disease (CWD) demonstrated that Norway and North America cervids are infected with distinct prion strains that respond differently to naturally occurring amino acid variation at residue 226 of the prion protein. Here we performed transmissions in gene-targeted mice to investigate the properties of prions causing newly emergent CWD in moose in Finland. Although CWD prions from Finland and Norway moose had comparable responses to primary structural differences at residue 226, other distinctive criteria, including transmission kinetics, patterns of neuronal degeneration, and conformational features of prions generated in the brains of diseased mice, demonstrated that the strain properties of Finland moose CWD prions are different from those previously characterized in Norway CWD. Our findings add to a growing body of evidence for a diverse portfolio of emergent strains in Nordic countries that are etiologically distinct from the comparatively consistent strain profile of North America CWD.
Keywords: CWD; Finland; Gt; Nordic; Norway; Tg; chronic wasting disease; gene-targeted; mice; moose; prions; transgenic.
Figures

Similar articles
-
Adaptive selection of a prion strain conformer corresponding to established North American CWD during propagation of novel emergent Norwegian strains in mice expressing elk or deer prion protein.PLoS Pathog. 2021 Jul 26;17(7):e1009748. doi: 10.1371/journal.ppat.1009748. eCollection 2021 Jul. PLoS Pathog. 2021. PMID: 34310663 Free PMC article.
-
Norwegian moose CWD induces clinical disease and neuroinvasion in gene-targeted mice expressing cervid S138N prion protein.PLoS Pathog. 2024 Jul 1;20(7):e1012350. doi: 10.1371/journal.ppat.1012350. eCollection 2024 Jul. PLoS Pathog. 2024. PMID: 38950080 Free PMC article.
-
Heterogeneity of pathological prion protein accumulation in the brain of moose (Alces alces) from Norway, Sweden and Finland with chronic wasting disease.Vet Res. 2023 Sep 8;54(1):74. doi: 10.1186/s13567-023-01208-3. Vet Res. 2023. PMID: 37684668 Free PMC article.
-
Chronic wasting disease in Europe: new strains on the horizon.Acta Vet Scand. 2021 Nov 25;63(1):48. doi: 10.1186/s13028-021-00606-x. Acta Vet Scand. 2021. PMID: 34823556 Free PMC article. Review.
-
Emergence of CWD strains.Cell Tissue Res. 2023 Apr;392(1):135-148. doi: 10.1007/s00441-022-03688-9. Epub 2022 Oct 6. Cell Tissue Res. 2023. PMID: 36201049 Free PMC article. Review.
Cited by
-
Monitoring of chronic wasting disease (CWD) (IV).EFSA J. 2023 Apr 17;21(4):e07936. doi: 10.2903/j.efsa.2023.7936. eCollection 2023 Apr. EFSA J. 2023. PMID: 37077299 Free PMC article.
-
Minor prion substrains overcome transmission barriers.mBio. 2024 Nov 13;15(11):e0272124. doi: 10.1128/mbio.02721-24. Epub 2024 Oct 23. mBio. 2024. PMID: 39440977 Free PMC article.
-
Variation in the prion protein gene (PRNP) open reading frame sequence in French cervids.Vet Res. 2024 Sep 3;55(1):105. doi: 10.1186/s13567-024-01362-2. Vet Res. 2024. PMID: 39227993 Free PMC article.
-
Cases of Creutzfeldt-Jakob disease in young individuals: open questions regarding aetiology.Front Cell Neurosci. 2025 Apr 14;19:1571662. doi: 10.3389/fncel.2025.1571662. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40297707 Free PMC article. No abstract available.
-
Transmission of Norwegian reindeer CWD to sheep by intracerebral inoculation results in an unusual phenotype and prion distribution.Vet Res. 2024 Jul 29;55(1):94. doi: 10.1186/s13567-024-01350-6. Vet Res. 2024. PMID: 39075607 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

