Multicenter Validation of Abbreviated MRI for Detecting Early-Stage Hepatocellular Carcinoma
- PMID: 36692401
- PMCID: PMC10102624
- DOI: 10.1148/radiol.220917
Multicenter Validation of Abbreviated MRI for Detecting Early-Stage Hepatocellular Carcinoma
Abstract
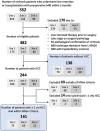
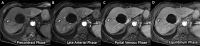
Background Abbreviated MRI is a proposed paradigm shift for hepatocellular carcinoma (HCC) surveillance, but data on its performance are lacking for histopathologically confirmed early-stage HCC. Purpose To evaluate the sensitivity and specificity of dynamic contrast-enhanced abbreviated MRI for early-stage HCC detection, using surgical pathologic findings as the reference standard. Materials and Methods This retrospective study was conducted at three U.S. liver transplant centers in patients with cirrhosis who underwent liver resection or transplant between January 2009 and December 2019 and standard "full" liver MRI with and without contrast enhancement within 3 months before surgery. Patients who had HCC-directed treatment before surgery were excluded. Dynamic abbreviated MRI examinations were simulated from the presurgical full MRI by selecting the coronal T2-weighted and axial three-dimensional fat-suppressed T1-weighted dynamic contrast-enhanced sequences at precontrast, late arterial, portal venous, and delayed phases. Two abdominal radiologists at each center independently interpreted the simulated abbreviated examinations with use of the Liver Imaging Reporting and Data System version 2018. Patients with any high-risk liver observations (>LR-3) were classified as positive; otherwise, they were classified as negative. With liver pathologic findings as the reference standard for the presence versus absence of early-stage HCC, the sensitivity, specificity, and their 95% CIs were calculated. Logistic regression was used to identify factors associated with correct classification. Results A total of 161 patients with early-stage HCC (median age, 62 years [IQR, 58-67 years]; 123 men) and 138 patients without HCC (median age, 55 years [IQR, 47-63 years]; 85 men) were confirmed with surgical pathologic findings. The sensitivity and specificity of abbreviated MRI were 88.2% (142 of 161 patients) (95% CI: 83.5, 92.5) and 89.1% (123 of 138 patients) (95% CI: 84.4, 93.8), respectively. Sensitivity was lower for Child-Pugh class B or C versus Child-Pugh class A cirrhosis (64.1% vs 94.2%; P < .001). Conclusion With surgical pathologic findings as the reference standard, dynamic abbreviated MRI had high sensitivity and specificity for early-stage hepatocellular carcinoma detection in patients with compensated cirrhosis but lower sensitivity in those with decompensated cirrhosis. © RSNA, 2023 Supplemental material is available for this article. See also the editorial by Kim in this issue.
Conflict of interest statement
Figures


Comment in
-
The Feasibility of Abbreviated MRI for Active Surveillance of Hepatocellular Carcinoma.Radiology. 2023 Apr;307(2):e223113. doi: 10.1148/radiol.223113. Epub 2023 Jan 24. Radiology. 2023. PMID: 36692405 No abstract available.
Similar articles
-
Non-contrast abbreviated MRI for the detection of hepatocellular carcinoma in patients with Liver Imaging Reporting and Data System LR-3 and LR-4 observations in MRI.Br J Radiol. 2024 Oct 1;97(1162):1671-1682. doi: 10.1093/bjr/tqae140. Br J Radiol. 2024. PMID: 39115388 Free PMC article.
-
Gadoxetate-enhanced Abbreviated MRI for Hepatocellular Carcinoma Surveillance: Preliminary Experience.Radiol Imaging Cancer. 2019 Nov 29;1(2):e190010. doi: 10.1148/rycan.2019190010. eCollection 2019 Nov. Radiol Imaging Cancer. 2019. PMID: 33778680 Free PMC article.
-
Abbreviated-protocol screening MRI vs. complete-protocol diagnostic MRI for detection of hepatocellular carcinoma in patients with cirrhosis: An equivalence study using LI-RADS v2018.J Magn Reson Imaging. 2020 Feb;51(2):415-425. doi: 10.1002/jmri.26835. Epub 2019 Jun 18. J Magn Reson Imaging. 2020. PMID: 31209978
-
Abbreviated MRI for Hepatocellular Carcinoma Screening and Surveillance.Radiographics. 2020 Nov-Dec;40(7):1916-1931. doi: 10.1148/rg.2020200104. Radiographics. 2020. PMID: 33136476 Free PMC article. Review.
-
Clinical outcomes of patients with Liver Imaging Reporting and Data System 3 or Liver Imaging Reporting and Data System 4 observations in patients with cirrhosis: A systematic review.Liver Transpl. 2022 Dec;28(12):1865-1875. doi: 10.1002/lt.26562. Epub 2022 Sep 5. Liver Transpl. 2022. PMID: 35980600 Free PMC article.
Cited by
-
Gadoxetic acid disodium (Gd-EOB-DTPA) contrast-enhanced abbreviated magnetic resonance imaging (MRI) for hepatocellular carcinoma surveillance in at-risk patients: a multi-center study in China.Quant Imaging Med Surg. 2024 Dec 5;14(12):8520-8537. doi: 10.21037/qims-24-941. Epub 2024 Oct 24. Quant Imaging Med Surg. 2024. PMID: 39698720 Free PMC article.
-
Natural History of Indeterminate Liver Nodules in Patients With Advanced Liver Disease: A Multicenter Retrospective Cohort Study.Am J Gastroenterol. 2024 Nov 1;119(11):2251-2258. doi: 10.14309/ajg.0000000000002827. Epub 2024 Apr 30. Am J Gastroenterol. 2024. PMID: 38686922
-
Abbreviated magnetic resonance imaging in hepatocellular carcinoma surveillance: A review.Indian J Gastroenterol. 2024 Dec;43(6):1090-1098. doi: 10.1007/s12664-023-01511-z. Epub 2024 Mar 9. Indian J Gastroenterol. 2024. PMID: 38460056 Review.
-
Hepatocellular Carcinoma Prevention in the Era of Hepatitis C Elimination.Int J Mol Sci. 2023 Sep 21;24(18):14404. doi: 10.3390/ijms241814404. Int J Mol Sci. 2023. PMID: 37762706 Free PMC article. Review.
-
National Liver Cancer Screening Trial (TRACER) study protocol.Hepatol Commun. 2024 Nov 4;8(11):e0565. doi: 10.1097/HC9.0000000000000565. eCollection 2024 Nov 1. Hepatol Commun. 2024. PMID: 39495136 Free PMC article.
References
-
- Llovet JM , Kelley RK , Villanueva A , et al. . Hepatocellular carcinoma . Nat Rev Dis Primers 2021. ; 7 ( 1 ): 6 . - PubMed
-
- Marrero JA , Kulik LM , Sirlin CB , et al. . Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases . Hepatology 2018. ; 68 ( 2 ): 723 – 750 . - PubMed
-
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma . J Hepatol 2018. ; 69 ( 1 ): 182 – 236 . [Published correction appears in J Hepatol 2019;70(4):817.] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

