The critical role of cardiolipin in metazoan differentiation, development, and maturation
- PMID: 36692477
- PMCID: PMC10238668
- DOI: 10.1002/dvdy.567
The critical role of cardiolipin in metazoan differentiation, development, and maturation
Abstract
Cardiolipins are phospholipids that are central to proper mitochondrial functioning. Because mitochondria play crucial roles in differentiation, development, and maturation, we would also expect cardiolipin to play major roles in these processes. Indeed, cardiolipin has been implicated in the mechanism of three human diseases that affect young infants, implying developmental abnormalities. In this review, we will: (1) Review the biology of cardiolipin; (2) Outline the evidence for essential roles of cardiolipin during organismal development, including embryogenesis and cell maturation in vertebrate organisms; (3) Place the role(s) of cardiolipin during embryogenesis within the larger context of the roles of mitochondria in development; and (4) Suggest avenues for future research.
Keywords: Tafazzin; mitochondria; oxidative phosphorylation; phospholipids; signaling.
© 2023 American Association for Anatomy.
Conflict of interest statement
Figures
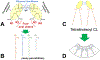



Similar articles
-
Cardiolipin remodeling and the function of tafazzin.Biochim Biophys Acta. 2013 Mar;1831(3):582-8. doi: 10.1016/j.bbalip.2012.11.007. Epub 2012 Nov 28. Biochim Biophys Acta. 2013. PMID: 23200781 Review.
-
Metabolism and function of mitochondrial cardiolipin.Prog Lipid Res. 2014 Jul;55:1-16. doi: 10.1016/j.plipres.2014.04.001. Epub 2014 Apr 24. Prog Lipid Res. 2014. PMID: 24769127 Review.
-
Cardiolipins and biomembrane function.Biochim Biophys Acta. 1992 Mar 26;1113(1):71-133. doi: 10.1016/0304-4157(92)90035-9. Biochim Biophys Acta. 1992. PMID: 1550861 Review.
-
Tafazzin Mutation Affecting Cardiolipin Leads to Increased Mitochondrial Superoxide Anions and Mitophagy Inhibition in Barth Syndrome.Cells. 2020 Oct 21;9(10):2333. doi: 10.3390/cells9102333. Cells. 2020. PMID: 33096711 Free PMC article.
-
Cardiolipin provides an essential activating platform for caspase-8 on mitochondria.J Cell Biol. 2008 Nov 17;183(4):681-96. doi: 10.1083/jcb.200803129. Epub 2008 Nov 10. J Cell Biol. 2008. PMID: 19001123 Free PMC article.
Cited by
-
Biallelic PTPMT1 variants disrupt cardiolipin metabolism and lead to a neurodevelopmental syndrome.Brain. 2025 Feb 3;148(2):647-662. doi: 10.1093/brain/awae268. Brain. 2025. PMID: 39279645 Free PMC article.
-
The Role of Cardiolipin in Mitochondrial Function and Neurodegenerative Diseases.Cells. 2024 Mar 30;13(7):609. doi: 10.3390/cells13070609. Cells. 2024. PMID: 38607048 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

