Enzymatic Conversion of CO2: From Natural to Artificial Utilization
- PMID: 36692850
- PMCID: PMC10176493
- DOI: 10.1021/acs.chemrev.2c00581
Enzymatic Conversion of CO2: From Natural to Artificial Utilization
Abstract
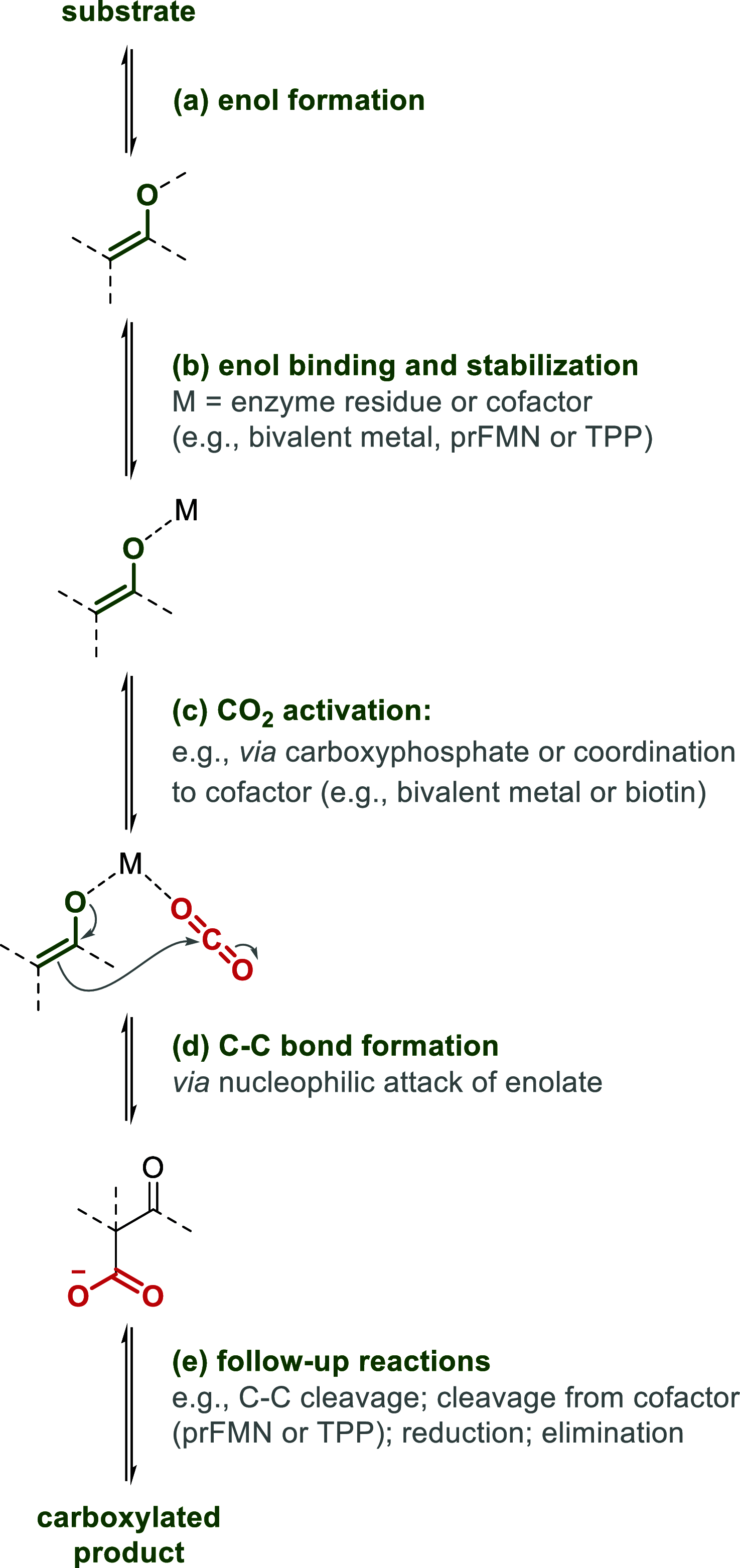
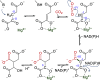
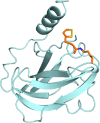
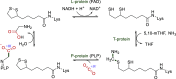
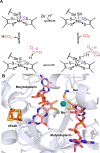
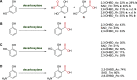
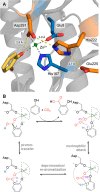
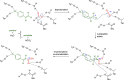
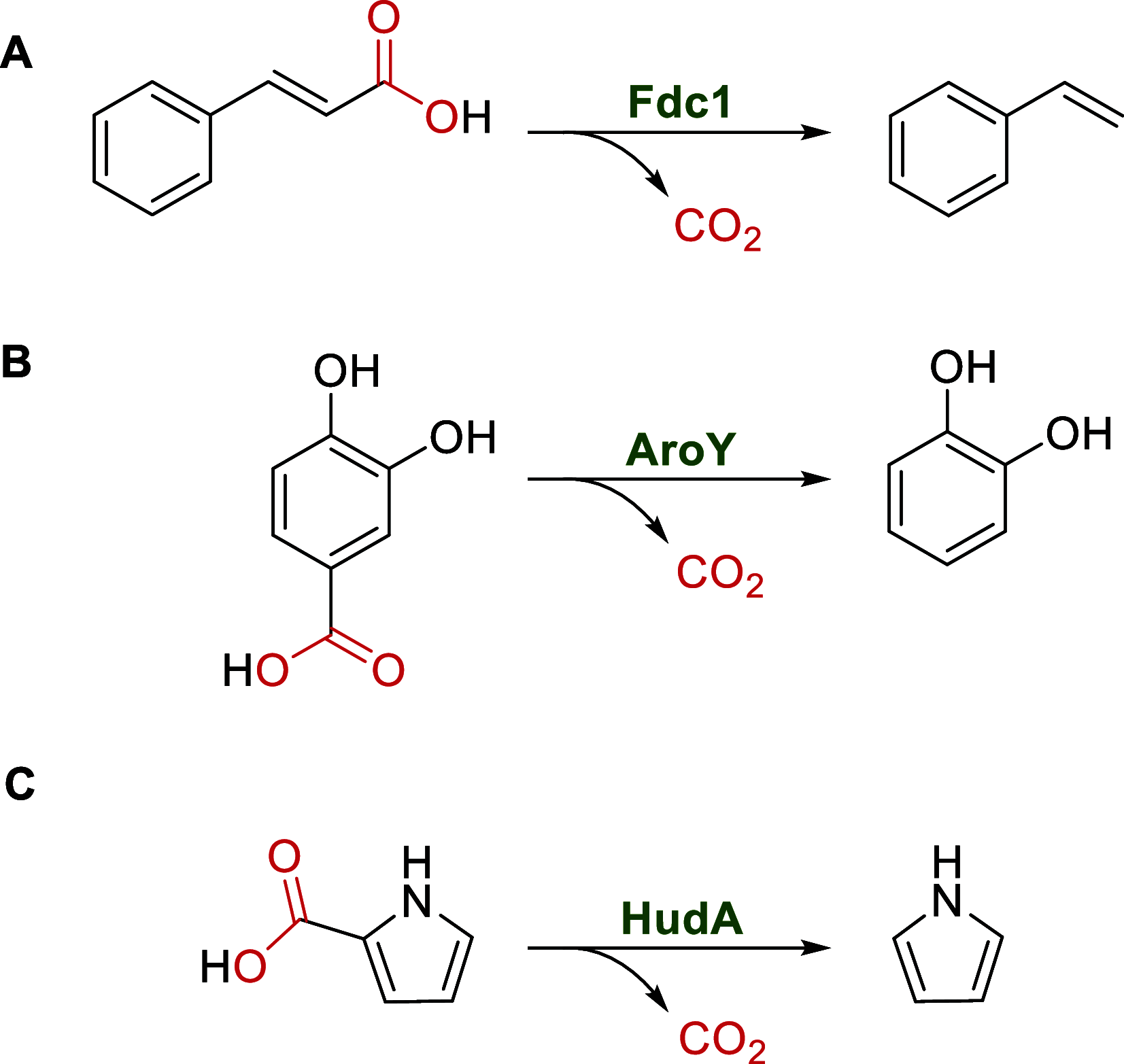
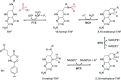
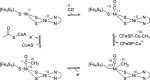
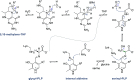
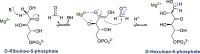
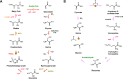
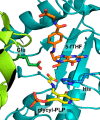
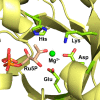
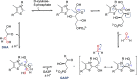
Enzymatic carbon dioxide fixation is one of the most important metabolic reactions as it allows the capture of inorganic carbon from the atmosphere and its conversion into organic biomass. However, due to the often unfavorable thermodynamics and the difficulties associated with the utilization of CO2, a gaseous substrate that is found in comparatively low concentrations in the atmosphere, such reactions remain challenging for biotechnological applications. Nature has tackled these problems by evolution of dedicated CO2-fixing enzymes, i.e., carboxylases, and embedding them in complex metabolic pathways. Biotechnology employs such carboxylating and decarboxylating enzymes for the carboxylation of aromatic and aliphatic substrates either by embedding them into more complex reaction cascades or by shifting the reaction equilibrium via reaction engineering. This review aims to provide an overview of natural CO2-fixing enzymes and their mechanistic similarities. We also discuss biocatalytic applications of carboxylases and decarboxylases for the synthesis of valuable products and provide a separate summary of strategies to improve the efficiency of such processes. We briefly summarize natural CO2 fixation pathways, provide a roadmap for the design and implementation of artificial carbon fixation pathways, and highlight examples of biocatalytic cascades involving carboxylases. Additionally, we suggest that biochemical utilization of reduced CO2 derivates, such as formate or methanol, represents a suitable alternative to direct use of CO2 and provide several examples. Our discussion closes with a techno-economic perspective on enzymatic CO2 fixation and its potential to reduce CO2 emissions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



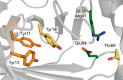
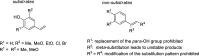



References
-
- Wiltschi B.; Cernava T.; Dennig A.; Galindo Casas M.; Geier M.; Gruber S.; Haberbauer M.; Heidinger P.; Herrero Acero E.; Kratzer R.; et al. Enzymes Revolutionize the Bioproduction of Value-Added Compounds: From Enzyme Discovery to Special Applications. Biotechnol. Adv. 2020, 40, 107520. 10.1016/j.biotechadv.2020.107520. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

