The Effects of HIV Infection on the Immune Response to Malaria Among Pregnant Women in Kumba, Southwest Cameroon: Protocol for a Cross-sectional Study
- PMID: 36692923
- PMCID: PMC9906321
- DOI: 10.2196/38213
The Effects of HIV Infection on the Immune Response to Malaria Among Pregnant Women in Kumba, Southwest Cameroon: Protocol for a Cross-sectional Study
Abstract
Background: Malaria and HIV, 2 of the world's deadliest diseases, share a lot of territory in sub-Saharan Africa.
Objective: This study seeks to investigate the effect of HIV on the immune response to malaria infection among pregnant women in Kumba in the southwest region (SWR) of Cameroon. The study aims to determine the prevalence of malaria infection, assess the occurrence of Plasmodium falciparum genetic diversity, and evaluate the antibody (immunoglobulin [Ig]G and IgM: apical membrane antigen-1 [AMA1], merozoite surface protein [MSP]1, MSP2, MSP3, and erythrocyte-binding antigen [EBA]175) and cytokine (interleukin [IL]-10, tumor necrosis factor alpha [TNF-α], and interferon gamma [IFNγ]) response to malaria infection among pregnant women with and without HIV in Kumba.
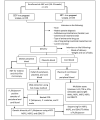
Methods: The study will be a hospital-based cross-sectional design that will run from March 2022 to February 2023. It will recruit pregnant women with and without HIV who are in their third trimester of pregnancy. The study will be carried out in 5 health institutions in Kumba: General Hospital Kumba, Presbyterian Hospital Kumba, District Hospital Kumba-town, Kossala Integrated Health Center Kumba, and Catholic Hospital Kumba. About 3 mL of the mother's venous blood, placental blood, and baby cord blood will be collected from each pregnant women at the point of delivery. Microscopy, rapid diagnostic tests (RDTs), and nested polymerase chain reaction (PCR) will be performed to identify the malaria parasite in all the samples, and nested PCR targeting the different genetic diversity markers for P. falciparum will also be performed. Furthermore, sequencing will be performed to study the nucleotide sequence of different alleles, and the genetic diversity of the alleles responsible for malaria infection among pregnant women will be assessed. A multiplex assay will be conducted to analyze the peripheral blood plasma and cord blood plasma for the cytokine and total antibody response to malaria infection among pregnant women with and without HIV. The questionnaire for data collection will be pretested at the Kumba District Hospital, and ethical clearance will be obtained from the University of Buea and the Regional Delegation of Public Health for the SWR. Data will be analyzed using SPSS Statistics and STATA. All P values <.05 will be considered statistically significant. BioEdit 7.0.0 software will be used to align the nucleotide sequences of different genes after sequencing. Phylogenetic tree searching will be conducted using the maximum-likelihood (ML) method in MEGA V6.0.
Results: The project started in March 2022 and will end in February 2023. Presently, three-fourth of the project funding has been disbursed to date. A total of 218 participants have been enrolled: 193 (88.5%) women without HIV and 25 (11.5%) women with HIV. Between February 2023 and March 2024, the following results will be ready for publication: maternal-neonatal malaria prevalence among pregnant women and babies in Kumba, the effect of HIV on (1) P. falciparum genetic diversity among pregnant women in Kumba, (2) the maternal and neonatal immune response to MSP1, MSP2, and EBA175 IgG antibody response to P. falciparum-caused malaria infection among pregnant women, and (3) the maternal and neonatal pro-inflammatory and anti-inflammatory cytokine response to malaria infection.
Conclusions: HIV infection increases the prevalence of malaria infection among pregnant women and also influences the genetic diversity of P. falciparum, with MSP1 alleles being the most prevalent. HIV infection also reduces the antibody response to malaria infection, as well as altering the level of pro-inflammatory and anti-inflammatory responses to malaria infection.
International registered report identifier (irrid): DERR1-10.2196/38213.
Keywords: Cameroon; HIV; allele; antibodies; co-infection; cytokines; immunity; malaria; pregnancy; prevalence.
©Bekindaka Ngemani Obase, Zeukeng Francis, Esemu Livo Forgu, Awanakam Honore, Jude Daiga Bigoga, Dickson S Nsagha. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 24.01.2023.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
Similar articles
-
Accuracy of One Step malaria rapid diagnostic test (RDT) in detecting Plasmodium falciparum placental malaria infection in women living in Yaoundé, Cameroon.Malar J. 2018 Dec 4;17(1):450. doi: 10.1186/s12936-018-2595-8. Malar J. 2018. PMID: 30514316 Free PMC article.
-
Impairment of a pregnant woman's acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1.Am J Trop Med Hyg. 1996;55(1 Suppl):42-9. doi: 10.4269/ajtmh.1996.55.42. Am J Trop Med Hyg. 1996. PMID: 8702036
-
Natural antibody responses to Plasmodium falciparum MSP3 and GLURP(R0) antigens are associated with low parasite densities in malaria patients living in the Central Region of Ghana.Parasit Vectors. 2017 Aug 23;10(1):395. doi: 10.1186/s13071-017-2338-7. Parasit Vectors. 2017. PMID: 28835262 Free PMC article.
-
Malaria and HIV infection in Mozambican pregnant women are associated with reduced transfer of antimalarial antibodies to their newborns.J Infect Dis. 2015 Mar 15;211(6):1004-14. doi: 10.1093/infdis/jiu547. Epub 2014 Sep 30. J Infect Dis. 2015. PMID: 25271267
-
Plasmodium falciparum msp1 and msp2 genetic diversity and allele frequencies in parasites isolated from symptomatic malaria patients in Bobo-Dioulasso, Burkina Faso.Parasit Vectors. 2018 May 30;11(1):323. doi: 10.1186/s13071-018-2895-4. Parasit Vectors. 2018. PMID: 29843783 Free PMC article.
References
-
- Elime FA, Nkenyi NR, Ako-Egbe L, Njunda A, Nsagha D. Malaria in pregnancy: prevalence and risk factors in the Mamfe health district, Cameroon. JAMMR. 2019 Jul 06;:1–11. doi: 10.9734/jammr/2019/v30i130161. - DOI
-
- McGregor D, Texeira da Silva E, Grignard L, Goncalves A, Vasileva H, Mabey D, Last A. The epidemiology of Plasmodium falciparum malaria in the Bijagos Islands of Guinea-Bissau. Am J Trop Med Hyg. 2021 Mar 29;104(6):2117–2122. doi: 10.4269/ajtmh.20-1029. https://europepmc.org/abstract/MED/33782209 tpmd201029 - DOI - PMC - PubMed
-
- Antonio-Nkondjio C, Ndo C, Njiokou F, Bigoga JD, Awono-Ambene P, Etang J, Ekobo AS, Wondji CS. Review of malaria situation in Cameroon: technical viewpoint on challenges and prospects for disease elimination. Parasit Vectors. 2019 Oct 26;12(1):501. doi: 10.1186/s13071-019-3753-8. https://parasitesandvectors.biomedcentral.com/articles/10.1186/s13071-01... 10.1186/s13071-019-3753-8 - DOI - DOI - PMC - PubMed
-
- Ejike B, Ohaeri C, Amaechi E, Ejike E, Okike-Osisiogu F, Irole-Eze OP, Belonwu A. Prevalence of falciparum malaria amongst pregnant women in Aba South Local Government Area, Abia State, Nigeria. Niger J Parasitol. 2017 Mar 29;38(1):48. doi: 10.4314/njpar.v38i1.9. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

