Neurons as stromal drivers of nervous system cancer formation and progression
- PMID: 36693322
- PMCID: PMC9883043
- DOI: 10.1016/j.devcel.2022.12.011
Neurons as stromal drivers of nervous system cancer formation and progression
Abstract
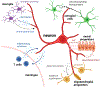
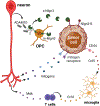
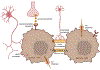
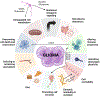
Similar to their pivotal roles in nervous system development, neurons have emerged as critical regulators of cancer initiation, maintenance, and progression. Focusing on nervous system tumors, we describe the normal relationships between neurons and other cell types relevant to normal nerve function, and discuss how disruptions of these interactions promote tumor evolution, focusing on electrical (gap junctions) and chemical (synaptic) coupling, as well as the establishment of new paracrine relationships. We also review how neuron-tumor communication contributes to some of the complications of cancer, including neuropathy, chemobrain, seizures, and pain. Finally, we consider the implications of cancer neuroscience in establishing risk for tumor penetrance and in the design of future anti-tumoral treatments.
Keywords: T cells; brain tumors; glioma; microglia; nerves; neuronal activity; neurotransmitter; synapse.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Similar articles
-
Update on connexins and gap junctions in neurons and glia in the mammalian nervous system.Brain Res Brain Res Rev. 2004 Dec;47(1-3):191-215. doi: 10.1016/j.brainresrev.2004.05.005. Brain Res Brain Res Rev. 2004. PMID: 15572172 Review.
-
Communication via gap junctions underlies early functional and beneficial interactions between grafted neural stem cells and the host.Proc Natl Acad Sci U S A. 2010 Mar 16;107(11):5184-9. doi: 10.1073/pnas.0915134107. Epub 2010 Feb 10. Proc Natl Acad Sci U S A. 2010. PMID: 20147621 Free PMC article.
-
Design principles of electrical synaptic plasticity.Neurosci Lett. 2019 Mar 16;695:4-11. doi: 10.1016/j.neulet.2017.09.003. Epub 2017 Sep 8. Neurosci Lett. 2019. PMID: 28893590 Free PMC article. Review.
-
Connexin-36 gap junctions mediate electrical coupling between ventral tegmental area GABA neurons.Synapse. 2006 Jul;60(1):20-31. doi: 10.1002/syn.20272. Synapse. 2006. PMID: 16575850
-
Pathways of neuron-astrocyte interactions and their possible role in neuroprotection.Eur Arch Psychiatry Clin Neurosci. 2001 Aug;251(4):159-69. doi: 10.1007/s004060170036. Eur Arch Psychiatry Clin Neurosci. 2001. PMID: 11697580 Review.
Cited by
-
A designer peptide against the EAG2-Kvβ2 potassium channel targets the interaction of cancer cells and neurons to treat glioblastoma.Nat Cancer. 2023 Oct;4(10):1418-1436. doi: 10.1038/s43018-023-00626-8. Epub 2023 Sep 11. Nat Cancer. 2023. PMID: 37697045
-
The Spatial Distribution of Brain Metastasis Is Determined by the Heterogeneity of the Brain Microenvironment.Hum Brain Mapp. 2024 Dec 15;45(18):e70103. doi: 10.1002/hbm.70103. Hum Brain Mapp. 2024. PMID: 39720836 Free PMC article.
-
Hsa_circRNA_0084043 promoting tumorigenesis in glioma through miR-577 sponging.Heliyon. 2023 Aug 17;9(9):e19219. doi: 10.1016/j.heliyon.2023.e19219. eCollection 2023 Sep. Heliyon. 2023. PMID: 37662721 Free PMC article.
-
Navigating glioblastoma complexity: the interplay of neurotransmitters and chromatin.Mol Biol Rep. 2024 Aug 17;51(1):912. doi: 10.1007/s11033-024-09853-3. Mol Biol Rep. 2024. PMID: 39153092 Free PMC article. Review.
-
Differential gene expression underlying epileptogenicity in patients with gliomas.Neurooncol Adv. 2024 Jun 21;6(1):vdae103. doi: 10.1093/noajnl/vdae103. eCollection 2024 Jan-Dec. Neurooncol Adv. 2024. PMID: 39022648 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

