Effect of early mobilisation on long-term cognitive impairment in critical illness in the USA: a randomised controlled trial
- PMID: 36693400
- PMCID: PMC10238598
- DOI: 10.1016/S2213-2600(22)00489-1
Effect of early mobilisation on long-term cognitive impairment in critical illness in the USA: a randomised controlled trial
Abstract
Background: Patients who have received mechanical ventilation can have prolonged cognitive impairment for which there is no known treatment. We aimed to establish whether early mobilisation could reduce the rates of cognitive impairment and other aspects of disability 1 year after critical illness.
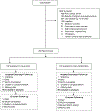
Methods: In this single-centre, parallel, randomised controlled trial, patients admitted to the adult medical-surgical intensive-care unit (ICU), at the University of Chicago (IL, USA), were recruited. Inclusion criteria were adult patients (aged ≥18 years) who were functionally independent and mechanically ventilated at baseline and within the first 96 h of mechanical ventilation, and expected to continue for at least 24 h. Patients were randomly assigned (1:1) via computer-generated permuted balanced block randomisation to early physical and occupational therapy (early mobilisation) or usual care. An investigator designated each assignment in consecutively numbered, sealed, opaque envelopes; they had no further involvement in the trial. Only the assessors were masked to group assignment. The primary outcome was cognitive impairment 1 year after hospital discharge, measured with a Montreal Cognitive Assessment. Patients were assessed for cognitive impairment, neuromuscular weakness, institution-free days, functional independence, and quality of life at hospital discharge and 1 year. Analysis was by intention to treat. This trial was registered with ClinicalTrials.gov, number NCT01777035, and is now completed.
Findings: Between Aug 11, 2011, and Oct 24, 2019, 1222 patients were screened, 200 were enrolled (usual care n=100, intervention n=100), and one patient withdrew from the study in each group; thus 99 patients in each group were included in the intention-to-treat analysis (113 [57%] men and 85 [43%] women). 65 (88%) of 74 in the usual care group and 62 (89%) of 70 in the intervention group underwent testing for cognitive impairment at 1 year. The rate of cognitive impairment at 1 year with early mobilisation was 24% (24 of 99 patients) compared with 43% (43 of 99) with usual care (absolute difference -19·2%, 95% CI -32·1 to -6·3%; p=0·0043). Cognitive impairment was lower at hospital discharge in the intervention group (53 [54%] 99 patients vs 68 [69%] 99 patients; -15·2%, -28·6 to -1·7; p=0·029). At 1 year, the intervention group had fewer ICU-acquired weaknesses (none [0%] of 99 patients vs 14 [14%] of 99 patients; -14·1%; -21·0 to -7·3; p=0·0001) and higher physical component scores on quality-of-life testing than did the usual care group (median 52·4 [IQR 45·3-56·8] vs median 41·1 [31·8-49·4]; p<0·0001). There was no difference in the rates of functional independence (64 [65%] of 99 patients vs 61 [62%] of 99 patients; 3%, -10·4 to 16·5%; p=0·66) or mental component scores (median 55·9 [50·2-58·9] vs median 55·2 [49·5-59·7]; p=0·98) between the intervention and usual care groups at 1 year. Seven adverse events (haemodynamic changes [n=3], arterial catheter removal [n=1], rectal tube dislodgement [n=1], and respiratory distress [n=2]) were reported in six (6%) of 99 patients in the intervention group and in none of the patients in the usual care group (p=0·029).
Interpretation: Early mobilisation might be the first known intervention to improve long-term cognitive impairment in ICU survivors after mechanical ventilation. These findings clearly emphasise the importance of avoiding delays in initiating mobilisation. However, the increased adverse events in the intervention group warrants further investigation to replicate these findings.
Funding: None.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
Comment in
-
Early mobilisation during critical illness: good for the body and brain.Lancet Respir Med. 2023 Jun;11(6):500-502. doi: 10.1016/S2213-2600(23)00011-5. Epub 2023 Jan 25. Lancet Respir Med. 2023. PMID: 36708729 No abstract available.
Similar articles
-
Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial.Lancet. 2009 May 30;373(9678):1874-82. doi: 10.1016/S0140-6736(09)60658-9. Epub 2009 May 14. Lancet. 2009. PMID: 19446324 Free PMC article. Clinical Trial.
-
Association of active mobilisation variables with adverse events and mortality in patients requiring mechanical ventilation in the intensive care unit: a systematic review and meta-analysis.Lancet Respir Med. 2024 May;12(5):386-398. doi: 10.1016/S2213-2600(24)00011-0. Epub 2024 Mar 18. Lancet Respir Med. 2024. PMID: 38513675
-
Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial.Lancet. 2016 Oct 1;388(10052):1377-1388. doi: 10.1016/S0140-6736(16)31637-3. Lancet. 2016. PMID: 27707496 Clinical Trial.
-
Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial.Lancet. 2015 Jul 4;386(9988):46-55. doi: 10.1016/S0140-6736(15)60690-0. Epub 2015 Apr 16. Lancet. 2015. PMID: 25892679 Clinical Trial.
-
Early mobilisation post-stroke: a systematic review and meta-analysis of individual participant data.Disabil Rehabil. 2022 Apr;44(8):1156-1163. doi: 10.1080/09638288.2020.1789229. Epub 2020 Jul 16. Disabil Rehabil. 2022. PMID: 32673130
Cited by
-
A New Era in Critical Care Trials: Linking ICU Practice to Long-Term Outcomes.Am J Respir Crit Care Med. 2024 Apr 1;209(7):782-784. doi: 10.1164/rccm.202402-0349ED. Am J Respir Crit Care Med. 2024. PMID: 38387023 Free PMC article. No abstract available.
-
Promoting optimal physical rehabilitation in ICU.Intensive Care Med. 2024 May;50(5):755-757. doi: 10.1007/s00134-024-07384-w. Epub 2024 Apr 2. Intensive Care Med. 2024. PMID: 38563898 No abstract available.
-
Implementation of a Quality Improvement Tool "Recover25" to Guide the Care of Patients Experiencing Prolonged Critical Illness: A Mixed-Method Feasibility Study.Crit Care Explor. 2025 May 13;7(5):e1265. doi: 10.1097/CCE.0000000000001265. eCollection 2025 May 1. Crit Care Explor. 2025. PMID: 40359353 Free PMC article.
-
High-concentration hydrogen inhalation mitigates sepsis-associated encephalopathy in mice by improving mitochondrial dynamics.CNS Neurosci Ther. 2024 Sep;30(9):e70021. doi: 10.1111/cns.70021. CNS Neurosci Ther. 2024. PMID: 39258790 Free PMC article.
-
We need to talk about adverse events during physical rehabilitation in critical care trials.EClinicalMedicine. 2024 Feb 1;68:102439. doi: 10.1016/j.eclinm.2024.102439. eCollection 2024 Feb. EClinicalMedicine. 2024. PMID: 38328754 Free PMC article. No abstract available.
References
-
- Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson LV. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160(1):50–6. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical